Abstract
Evidence for the Higgs boson, for which the Nobel Prize in physics was awarded in 2013, was discovered by the European Organization for Nuclear Research (CERN) in 2012, using the Large Hadron Collider (LHC). PIXEO BP-S exhibited superior resistance to both radiation and cryogenics and this polymer played a crucial role in the operation of the LHC, thus contributing to the Higgs boson discovery. Herein, we report the synthesis and characterization of various polyester imides for a cryogenic and radiation-resistant polyimide adhesive tape. These polyester imides (3) were synthesized from 2,2-bis(4-hydroxyphenyl)propanedibenzoate-3,3ʹ,4,4ʹ-tetracarboxylic acid anhydride (ESDA) and various diamines (1a–1l). All polymers showed high solubility in various solvents, especially 3a, which was synthesized from ESDA and 4,4ʹ-bis(3-aminophenoxy)diphenyl sulfone and dissolved even in ether solvents such as dioxane and dioxolane. Dynamic mechanical analysis and X-ray diffraction studies indicated that the excluded volume of the polyester imides had a length of 150 Å, and that this volume affected the thermoplastic properties and solubilities of the polymers. The degree of imidization of 3a dissolved in dimethyl sulfoxide was examined by 1H-Nuclear magnetic resonance(NMR) imaging utilizing a 15N-enriched amine model compound in conjunction with 1H–15N heteronuclear single quantum coherence 2-dimensional NMR and was found to be 99.5%.
Similar content being viewed by others
Introduction
In 2013, the Nobel Prize in Physics was awarded to Dr François Englert and Dr Peter W Higgs for their work on the Higgs boson.1 Evidence for the existence of this particle had been obtained the previous year by the European Organization for Nuclear Research (CERN), using the Large Hadron Collider (LHC).2
In the late 1990s, CERN announced that the Large Electron-Positron Collider, located 100 m below ground at a site approximately 27 km from Geneva, would be replaced by the LHC as part of their efforts to discover proof of the existence of the Higgs boson shown in Figure 1. At the time, CERN had a requirement for a polyimide film and a polyimide adhesive tape with good resistance to radiation and cryogenics. In response to this requirement, 24.5 MT of a polyimide adhesive tape and 66.5 MT of a polyimide film itself were provided to CERN between 2000 and 2005.
(a) The Large Hadron Collider at CERN (Home Page of High Energy Accelerator Research Organization (KEK), http://semrl.t.u-tokyo.ac.jp/supercom/85/85_1.html). (b) The cross section of LHC Dipole, and (c) polyimide tape lapping.
The use of a polyimide tape that can be adhered at low temperatures and pressures on the superconducting wires was important so as to avoid quench cracking phenomenon.3, 4 Towards this purpose, we successfully designed a series of solvent soluble thermoplastic polyester imides that could be coated on a base polyimide film. Upon wrapping of this coated film on to the cables, it exhibited good adherence when subjected to low pressure–temperature conditions. The polyimide tape was lapped on to the superconducting strands by a 50% overlap of each winding for thorough coverage and insulation. The robust property of this material allowed it to withstand radiation (alpha particles) and exposure to cryogenic temperatures (liquid helium flow) from the collider, thus meeting all requirements set by CERN.
As a result of the contribution of the polyimide adhesive tape to the discovery of the Higgs Boson, this product has been presented with the Award of the Society of Polymer Science, Japan (2014). Herein, we report the synthesis of polyester imides for a polyimide adhesive tape, as well as the characterization of these materials.
Polyester imides
Importance and novelty of polyester imides
Loncrini5 first reported the synthesis of polyester imides from 2,2-bis(4-hydroxyphenyl propanedibenzoate-3,3ʹ,4,4ʹ-tetracarboxylic acid anhydride (ESDA) via trimellitate acetate, bisphenol-A and various diamines in the late 1960s. Although Kurita6 has reviewed the subsequent progress of research into polyester imides, there have been only a few literature reports concerning the use of ESDA to date.
In the present study, the HOMO and LUMO energy levels of ESDA were calculated and compared with those of other dianhydrides. As can be seen from Table 1, it was found that the LUMO energy level of ESDA was lower than those of Da and Db (see Table 1 for the full names of all compounds), nearly equal to that of TMHQ, and higher than those of ODPA, BTDA, BPDA, and PMDA. It has been proposed that LUMO energy levels7 have a significant effect on the reactivities of dianhydrides during electrophilic attack (an SN2-type reaction) by diamines. Therefore, it follows that a lower LUMO energy level should result in higher reactivity. In keeping with this theory, ESDA was found to have moderate reactivity among the series of dianhydrides listed in Table 1. ESDA was thus considered as one of several potential dianhydrides for polyimide syntheses.
Syntheses of polyester imides
A series of polyester imides (3) were synthesized from the reaction of ESDA with various diamines (1a–1l) by a newly developed rapid thermal imidization method using polyamic acid intermediates (2). The chemical structures of the resulting polyester imides are presented in Figure 2. Each of these polymers showed high solubility in various solvents, especially 3a, which was synthesized from 4,4ʹ-bis(3-aminophenoxy) diphenyl sulfone and ESDA, and was soluble even in the ether solvents dioxane and dioxolane.
The imidization ratios of the polyester imides were determined by a newly developed method based on a 1H–15N heteronuclear single quantum coherence (HSQC) 2-dimensional (2D) nuclear magnetic resonance (NMR) imaging technique utilizing a 15N-enriched amine model compound.
Characteristics of polyester imides
Table 2 summarizes the solubilities of the polyester imides in various common solvents. It was found that 3a, 3c, 3d, 3e and 3f, all of which had ether linkages, ester linkages or were meta-substituted, showed solubility in THF and some other ether solvents. Compounds 3a and 3c were particularly soluble in dioxane and dioxolane, while 3l was also soluble in CHCl3.
Casting of 3a from a dioxolane solution generated a transparent, light yellow film with the properties summarized in Table 3.
Dynamic mechanical analysis (DMA) of the resulting polymers was also performed, and a typical DMA plot is presented in Figure 3. This plot shows that above its glass transition temperature (Tg) of 185 °C, the storage elastic modulus of the material (Eʹ) drops rapidly to a value of approximately 105. In the temperature range above its Tg, 3a thus exhibited excellent flow properties.
X-ray diffraction (XRD) crystalline structure analysis of ESDA was performed after obtaining a pure crystal from DMF solution. An ORTEP drawing of the ESDA repeat unit is shown in Figure 4. The high solubility of this polyester imide can be attributed to the bent structure of its repeat units. The 3D structure of 3i, composed of ESDA and 1i, was calculated with Gaussian 03 HF/STO-3G using the ORTEP data for ESDA, and is shown in Figure 5.
The DMA and XRD data indicated that the excluded volume of the polyester imide was 150 Å in length, and that this volume was associated with the unique thermoplastic properties and solubility of the polymer.8, 9 As a result, it can be said that the properties of polyester imide 3i result from its amorphous nature.10, 11
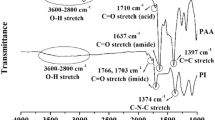
The determination of imidization ratios by infrared spectroscopy measurements has been reported, although not in a quantitative manner.12 Imidization ratios of polyimides in organic solvents have also been assessed using 1H-NMR spectroscopy, but there are associated difficulties in confirming the N–H peak positions.13 Therefore, it was necessary to develop a new method to assess the degree of imidization of these polyester imides. For this purpose, 3a was dissolved in DMSO and studied using a 15N-enriched amine model compound and 1H–15N HSQC 2D NMR.14, 15 In addition, a 19F-NMR method capable of elucidating the trifluoroamide-NH structures of the derivatized polymer was employed.
The chemical structure of the model compound A, composed of ESDA and aniline-15N, is shown in Figure 6, and the 1H-NMR spectra of A in its original and its imidized forms are presented in Figures 7a and b, respectively. These spectra confirmed that the N–H peak is found at 10.5 p.p.m. and the COO–H peak at 13.5 p.p.m. The 1H–15N HSQC spectrum of compound A is given in Figure 7c. From this, the N–H peak of compound A was found to appear at 10.4–10.6 p.p.m.
The 1H-NMR spectrum of 3a is shown in Figure 8.
In Figure 8, an N–H peak is observed at 10.5 p.p.m. and the protons of the aromatic group appear from 7 to 9 p.p.m.
From these data, the imidization ratio of 3a was calculated using Equation (1).

Here Aam and Aar represent the integration values of the N–H and aromatic protons. Based on Figure 8 and Equation (1), the imidization ratio of 3a was determined to be 99.5%.
Conclusion
Evidence for the existence of the Higgs boson, for which the Nobel Prize in Physics was awarded in 2013, was discovered by CERN in 2012, using the LHC. During this work, a polyester imide having both high radiation resistance and superior cryogenic properties, was adopted as the superconducting magnet insulation adhesive tape within the LHC. In this study, we synthesized various polyester imides, including 3a, and examined the properties of these polymers. Recently, in 2013, it was further confirmed during a maintenance and repair process of LHC that the PIXEO BP-S polyimide adhesive tape still retained its radiation and cryogenic temperature resistance as same as when it was received.16
References
Sogabe, K., Miyauchi, M., Kikuchi, T., Tsuji, H., Ida, J. & Furutani, H. Characteristics of polyesterimides consisting of 2,2-Bis(4-hydroxyphenyl)propane-dibenzoate-3,3',4,4'-tetracarboxylic acid anhydride (ESDA) and various diamines. Kobunshi Ronbunshu 70, 647–654 (2013).
Nihon Syoken Shinbun (Evening Edn, 15 October (2013).
Fessia, P., Kummer, H., Kuribayashi, H., Tommasini, D. & Van de Velde, F. Curing of LHC main dipole coils insulated with all polyimide PIXEO_ adhesive tape. IEEE Trans. Appl. Sup 16, 1782–1785 (2006).
LHC Project Document No. LHC-VCX-AP-0001 (2002).
Loncrini, D. F. Aromatic polyesterimides. J. Polym. Sci. 4, 1531–1541 (1966).
Kurita, K. Polymer Applications 37, 74–78 (1988).
Furutani, H., Ida, J., Tanaka, K. & Nagano, H. Novel soluble ethynyl-terminated ester-imide prepolymers: their syntheses and characteristics. High Perf. Polym. 12, 461–469 (2000).
Stewart, J. J. P. MOPAC version 6.0 (QCPE #455)
Hirano, T. MOPAC version 6.01 (JCPE P049)
Miyauchi, M. Development of novel asymmetric and addition-type imide resin with high solubility and excellent processability. Kobunshi Ronbunshu 69, 580–587 (2012).
Furutani, H. in Proceedings 21st Japan polyimides and aromatic polymers conference, recent progress of polyimides, 29-32 (Tokyo, 2013)
Karamanchevaa, V., Stefovb, B., Šoptrajanovb, D. G., Spasovad, E. & Assad, J. FTIR spectroscopy and FTIR microscopy of vacuum-evaporated polyimide thin films. Vib. Spectrosc. 19, 369–374 (1999).
Matsuura, T., Hasuda, Y., Nishi, S. & Yamada, N. Polyimide derived from 2,2'-bis(trifluoromethyl)-4,4'-diaminobiphenyl. 1. Synthesis and characterization of polyimides prepared with 2,2'-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride or pyromellitic dianhydride. Macromolecules 24, 5001–5005 (1991).
Bodenhausen, G. & Ruben, D. Natural abundance nitrogen-15 NMR by enhanced heteronuclear spectroscopy. J. Chem. Phys. Lett. 69, 185–189 (1980).
Grzesiek, S. & Bax, A. The importance of not saturating water in protein NMR. Application to sensitivity enhancement and NOE measurements. J. Am. Chem. Soc. 115, 12593 (1993).
Rossi, L. in Cryogenics and Superconductivity Society of Japan’s Presentation (University of Kyoto, 2013)
Acknowledgements
The authors would like to express their sincere appreciation and gratitude to Mr Hirosaku Nagano, CTO of the Kaneka Corporation, for his guidance, encouragement and advice, without which this work would not have been possible. They also wish to thank their colleagues at the Kaneka Corporation for their active collaboration during this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Furutani, H., Tsuji, H. & Sogabe, K. Synthesis and properties of polyester imides based on 2,2-bis(4-hydroxyphenyl)propanedibenzoate-3,3ʹ,4,4ʹ-tetracarboxylic acid dianhydride. Polym J 49, 587–591 (2017). https://doi.org/10.1038/pj.2017.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2017.24
This article is cited by
-
Preparation and properties of semi-alicyclic thermoplastic polyimide sheets from stereoisomeric hydrogenated pyromellitic dianhydrides and fluorinated diamine
Journal of Polymer Research (2024)
-
Preparation and properties of laterally tert-butyl-substituted cyclohexyl-containing polyimide alignment layers with high pretilt angles for potential applications in twisted-nematic TFT-LCDs
Journal of Polymer Research (2022)
-
Synthesis and characterization of two novel poly(amide-imide)s containing pendent (5-(4,5-diphenyl-1H-imidazol-2-yl)furan-2-yl)phenyl moieties and natural amino acids linkages for adsorption of Cu(II)
Polymer Bulletin (2020)