Abstract
An enzyme electrode was prepared by covalent immobilization of acid phosphatase (ACP) on a polyaniline/poly(acrylic acid) composite film (PANI/PAA film) deposited on an Au electrode and used as the anode of a fuel cell driven with L-ascorbic acid 2-phosphate (ASA2P). Biochemical and electrochemical properties of the enzyme electrode were compared with those of the one fabricated without the PANI/PAA film by enzyme immobilization on an Au electrode bearing a self-assembled monolayer formed with 3-mercaptopropionic acid. The former electrode was found superior to the latter in the quantity of immobilized ACP, the function to scavenge ascorbic acid (ASA), resulting from dephosphorylation of ASA2P by ACP and the electrochemical ability to oxidize ASA. The ASA2P fuel cells were constructed by use of the enzyme electrodes as anodes. The fuel cell using the enzyme electrode fabricated with the PANI/PAA film gave a maximum power output of 6.0 μW cm−2-anode, which was 70 times as large as that obtained with the cell using the electrode fabricated without the PANI/PAA film, and thus the power output was dependent on the performance of the enzyme electrode.
Similar content being viewed by others
Introduction
A wide variety of fuel cells have been developed as power sources. Among them, a direct methanol fuel cell (DMFC) has attracted great attention owing to its superior performance and been investigated actively for portable application over recent years.1 Nevertheless, the MFC still has some problems to be solved, such as the use of toxicant methanol as a fuel and precious metals as a catalyst. On the other hand, an ascorbic acid (ASA) fuel cell (ASAFC) has been investigated, in which ASA has been used as a fuel in the form of aqueous solution.2, 3, 4, 5 ASA, a safe substance well known as vitamin C, has electrochemical activity even in the absence of any precious metal catalysts. Thus, the ASAFC has the merits to overcome the problems accompanying the DMFC. However, ASA has poor storage stability in an aqueous solution and this often prevents ASA from being used as a fuel.6 In most cases, therefore, the ASAFC has been driven by use of sulfuric acid as an electrolyte, which is unfavorable for portable devices from the viewpoint of safety.
In a preliminary study, the authors proposed a novel system combining biocatalytic dephosphorylation of L-ascorbic acid 2-phosphate (ASA2P) with acid phosphatase (ACP) and electrochemical oxidation of the resulting ASA by use of the ACP-immobilized electrode.7 This system can be applied to a fuel cell driven with ASA2P instead of ASA. ASA2P is a safe material similarly to ASA and, besides, has a high stability in an aqueous solution.8 By use of ASA2P as a fuel, biocompatible buffer solutions can serve as the electrolyte to secure the safety of the fuel cell. However, ASA2P cannot be oxidized easily because its phosphate group protects the conversion to the oxidized form of ASA. For this reason, ACP was immobilized on the electrode for conversion of ASA2P to ASA. In this manner, an oxidation current was obtained with the ACP-immobilized electrode in buffer solutions containing ASA2P.
As to the development of the anode of the ASA2P fuel cell, the methods of fabricating the enzyme electrodes for amperometric biosensors and other biofuel cells can be applied,9, 10, 11, 12 in which the use of conducting polymers is helpful. The authors reported in a previous paper13 that the film composed of polyaniline (PANI) and poly(acrylic acid) (PAA) showed both conductivity and electrochemical activity. The PANI/PAA film has a part of the carboxyl groups of PAA on its surface and, therefore, enzymes can be immobilized covalently on the surface through amide linkages.14, 15, 16 In addition, PANI is known to catalyze the electrochemical oxidation of ASA.17, 18, 19 Thus, the PANI/PAA film is a promising candidate for the conducting component of the enzyme electrode for bioelectrochemical oxidation of ASA2P.
In the present study, the PANI/PAA film was deposited on an Au electrode, and ACP was immobilized covalently on the film through amide linkages by the condensation reaction between the amino groups of ACP and the carboxyl groups on the film. The ACP-immobilized electrode fabricated with the PANI/PAA film in this way (PANI/PAA-ACP electrode) was employed as an anode of the ASA2P fuel cell. The performance of the fuel cell was compared with that of the cell equipped with an enzyme electrode fabricated without the PANI/PAA film by immobilizing ACP on an Au electrode bearing a self-assembled monolayer formed with 3-mercaptopropionic acid (MPA).
Experimental procedure
Materials
Aniline obtained from Nacalai Tesque, Inc. (Kyoto, Japan) was distilled under reduced pressure and stored under nitrogen at −20 °C until use. PAA (molecular weight 250 000) and a trisodium salt of ASA2P were purchased from Wako Pure Chemical Ind. (Osaka, Japan). The ASA2P was purified by recrystallization from water and methanol at room temperature before use. ASA, MPA, ACP (EC 3. 1. 3. 2, from wheat germ) and N-hydroxysuccinimide were obtained from Nacalai Tesque, Inc. N-cyclohexyl-N-(2-morpholinoethyl) carbodiimide metho-p-toluenesulfonate (CMC, used as a condensing reagent) and Nafion 115 (polyelectrolyte membrane with a thickness of 0.005 inch) were purchased from Sigma-Aldrich, Inc. (St Louis, MO, USA). Pt-loaded carbon paper (EC-10-05-7) was supplied by ElectroChem, Inc. (Woburn, MA, USA). Other chemicals were of analytical grade, which were used without further purification. All aqueous solutions were prepared with distilled water.
Electrochemical apparatus
Electrochemical polymerization was carried out in a conventional three-electrode cell equipped with a potentiostat/galvanostat (μAutolab Type III, Eco Chemie, Utrecht, The Netherlands). An Au film deposited on a quartz plate was used as a working electrode (0.25 cm2). Before use, the Au electrode was cleaned with a piranha solution (H2SO4:30% H2O2=3:1). A Pt plate and a saturated calomel electrode were used as a counter electrode and a reference electrode, respectively.
Electrochemical measurements were carried out with the same apparatus as described above, except that an Ag/AgCl electrode was used as a reference electrode.
Preparation of ACP-immobilized electrode
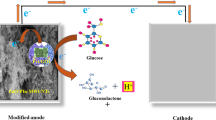
To begin with, the PANI/PAA film was prepared by electrochemical polymerization of aniline in a 0.5 M H2SO4 solution containing 0.5 M aniline and 25 mg ml−1 PAA as described in a previous publication:13 After purging the solution with N2, the polymerization was carried out by means of cyclic voltammetry from −0.4 to 0.9 V vs a saturated calomel electrode at a scan rate of 0.05 V s−1. The potential scan was repeated until the amount of passed charge reached 35 mC. The resulting PANI/PAA films were washed with 0.5 M H2SO4 and then with distilled water. Then, ACP was immobilized on the PANI/PAA film by the reaction shown in Scheme 1. The PANI/PAA film was immersed in a 2 ml aqueous solution of 100 mg CMC and 15 mg N-hydroxysuccinimide for 20 min to activate the carboxyl groups on the film by esterification with N-hydroxysuccinimide. After washing with distilled water, 15 μl of 5 mg ml−1 aqueous solution of ACP was dropped onto the PANI/PAA film, and the film was left at room temperature for 30 min being covered with a Petri dish for prevention of water evaporation. The PANI/PAA film treated thus, that is, the PANI/PAA-ACP electrode, was washed with 1 ml of 0.5 M acetate buffer of pH 5.0 and then with distilled water. The buffer solution containing unbound ACP was recovered, and the amount of immobilized ACP was estimated from the difference in enzyme activity between the ACP solution recovered and that prepared for the immobilization.
For comparison, an ACP-immobilized electrode of another type was fabricated, without the PANI/PAA film, by attaching ACP to the self-assembled monolayer formed with MPA on an Au electrode in the following manner. An Au electrode was immersed in a 10 mM ethanol solution of MPA for 30 min at room temperature and then washed with distilled water. Subsequently, ACP was immobilized on the electrode in the same way as the case of that bearing the PANI/PAA film. The electrode treated thus, that is, the MPA-ACP electrode, was stored, as well as the PANI/PAA-ACP electrode, in the acetate buffer at 4 °C until use.
Biochemical measurement
Activity of ACP was determined by a colorimetric method involving the reaction of phosphate ions produced by ACP-catalyzed dephosphorylation of ASA2P with molybdate ions to yield molybdophosphate ions.20 The dephosphorylation with ACP was conducted in 0.1 M acetate buffer of pH 5.0 containing 10 mM ASA2P at 25 °C. The activity was evaluated from absorbance at 700 nm owing to reduction of the molybdophosphate ions. The absorbance was measured on a Shimadzu UV-3100PC spectrometer.
Electrochemical measurements
Redox behavior of the PANI/PAA film was examined by cyclic voltammetry in 0.1 M acetate buffer of pH 5.0 at a scan rate of 10 mV s−1. Electrochemical properties of the PANI/PAA film, and the PANI/PAA-ACP and MPA-ACP electrodes were investigated by chronoamperometry. The measurements were carried out over the potential range from −0.2 to 0.4 vs Ag/AgCl in the buffer containing 10 mM of ASA or ASA2P. In advance, N2 was passed through the buffer for 20 min. Both the cyclic voltammetry and chronoamperometry were carried out at 25 °C.
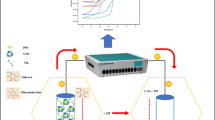
The ASA2P fuel cell was assembled as illustrated in Figure 1a, which was equipped with the PANI/PAA-ACP or MPA-ACP electrode as an anode and Pt-loaded carbon paper as a cathode. The anodic and cathodic compartments were isolated by Nafion 115. The Pt-loaded carbon was bonded to the Nafion membrane by hot pressing. A stainless steel mesh was used as a current collector in the cathodic compartment. The anodic compartment was filled with a 0.1 M acetate buffer containing 10 mM ASA2P, which was saturated with dissolved N2 by bubbling. The power outputs were determined by measuring currents at arbitrary potentials at 25 °C under atmospheric pressure.
Results and discussion
Electrochemical properties of the PANI/PAA film
Figure 1b shows a series of redox reactions in the ASA2P fuel cell, in which ASA2P is oxidized bioelectrochemically in the anodic compartment, and O2 is fed to the cathodic compartment through the stainless steel mesh and the carbon paper and reduced to H2O. In this system, the PANI/PAA film on the anode has important roles in both providing ACP-immobilizing sites and oxidizing the ASA produced by biocatalytic dephosphorylation of ASA2P. It was confirmed, in accordance with the previous study,13 that the PANI/PAA film had a PAA content of 18.2% and a conductivity of 5.6 × 10−2S cm−1. As shown by the cyclic voltammograms in Figure 2, the PANI/PAA film had an electrochemical stability and gave definite peaks reflecting the redox process of the PANI protonated by the carboxyl groups of coexisting PAA, whereas a PANI film gave no definite redox peaks. The intensity of the redox peaks was hardly decreased by successive potential scans and, therefore, the elimination of PAA from the PANI/PAA film was considered to be neglected. A possible reason for the irreversible voltammograms in Figure 2 is that the redox process between leucoemeraldine and pernigraniline is late compared with potential scan, which may be relevant to the thickness of the film. On the other hand, when 10 mM of ASA was added to the same system that yielded the result in Figure 2, an increase in the oxidation current was observed, as shown in Figure 3. This current increase can be attributed to electrochemical oxidation of ASA on the film.
Electrochemical oxidation of ASA was examined with the PANI/PAA and PANI films at various applied potentials. Figure 4 shows the relation between the oxidation current and the potential. It can be seen that the ASA-oxidizing behavior of the PANI/PAA film is almost the same as that of the PANI film without PAA. The onset potential of the oxidation was found to be ca. 0 V vs Ag/AgCl for both the electrodes. At a potential higher than 0.2 V, the oxidation currents leveled off to become 0.6 mA cm−2. Thus, the PANI/PAA film and PANI film had the electrocatalytic activity to oxidize ASA. According to previous studies,17, 18 this catalytic process is considered essentially to be chemical reduction of oxidized PANI due to ASA followed by electrochemical oxidation of reduced PANI. It seems, in addition, that the PAA in the PANI/PAA film does not affect the electrocatalytic activity of PANI at all. Therefore, the number of catalytic sites on the PANI/PAA film is almost the same as that on the PANI film.
Bioelectrochemical oxidation of ASA2P with the PANI/PAA-ACP electrode
In the present ASA2P fuel cell system, both the amount and activity of the ACP on its anode are important parameters relevant to the conversion of ASA2P to ASA. Table 1 shows the amounts and activities of the immobilized ACP on the PANI/PAA-ACP and MPA-ACP electrodes. The amount of ACP on the PANI/PAA-ACP electrode was much larger than that on the MPA-ACP electrode and, accordingly, a higher enzyme activity was observed for the former electrode than for the latter. It has been demonstrated that the PANI/PAA film has a fibrous network structure.13 Taking this morphology into account, the large amount of ACP on the PANI/PAA-ACP electrode can be attributed to a large surface area of the PAA/PANI film. Because of such a complicated surface structure, there is a possibility that ACP was adsorbed physically on the film. For the purpose of confirming this possibility, immobilization of ACP was attempted without activation of the carboxyl groups on the film by esterification with N-hydroxysuccinimide. As a result, it was found that the amount and activity of the immobilized ACP were 14.5 μg and 0.17 mU, respectively, which were smaller than those of the ACP immobilized by the condensation reaction shown in Scheme 1. This result suggests that the carboxyl groups on the film participated in the covalent immobilization of ACP. Thus, the complicated surface structure and large surface area of the film are closely related to the amount and condition of immobilized ACP and, therefore, to the electrochemical performance of the PANI/PAA-ACP electrode and the stability of the fuel cell equipped with it.
Figure 5 shows the effect of applied potential on the oxidation currents measured with the PANI/PAA-ACP and MPA-ACP electrodes in the acetate buffer containing ASA2P. The PANI/PAA-ACP electrode gave the oxidation current clearly at a potential above 0.1 V vs Ag/AgCl, whereas the current observed with the MPA-ACP electrode was extremely small. It should be noted that the onset potential of the oxidation with the PANI/PAA-ACP electrode was 0.1 V lower than that observed with the MPA-ACP electrode. Thus, the PANI/PAA-ACP electrode functioned well in bioelectrochemical oxidation of ASA2P.
In the oxidation of ASA2P with the ACP-immobilized electrode, the ASA produced by dephosphorylation of ASA2P must diffuse from the vicinity of the ACP onto the electrode surface to be oxidized. Needless to say, the ASA diffusing away into bulk solution cannot be oxidized electrochemically. It follows, therefore, that the efficiency of electrochemical oxidation of ASA is an important factor to determine the performance of the electrode. Herein, the efficiency was estimated for the PANI/PAA-ACP and MPA-ACP electrodes based on the theoretical value of the oxidation current calculated from the activity of immobilized ACP. Table 2 lists these parameters at 0.2 V vs Ag/AgCl, in which the oxidation efficiency means the percentage of the electrochemically oxidized ASA among the total ASA resulting from dephosphorylation of ASA2P. The oxidation efficiency for the PANI/PAA-ACP electrode was ca. 86%, whereas that for the MPA-ACP electrode was only 20%. The high efficiency for the PANI/PAA-ACP electrode can be attributed to the fact that the PANI/PAA film has a dense network structure,13 which prevents the ASA from diffusing away into bulk solution. In addition, it seems that the immobilized ACP hardly interfered with the oxidation of ASA molecules on the PANI/PAA-ACP electrode and, therefore, the performance of the electrode can be improved by more efficient conversion of ASA2P to ASA.
Application of the PANI/PAA-ACP electrode as an anode of the ASA2P fuel cell
The ASA2P fuel cell was constructed by use of the PANI/PAA-ACP or MPA-ACP electrode and Pt-loaded carbon paper as an anode and a cathode, respectively. In advance, O2 reduction was examined with the Pt-loaded carbon paper to confirm that the obtained current was much larger than that given by ASA2P oxidation with the PANI/PAA-ACP or MPA-ACP electrode. Then, these two electrodes were compared with each other from the viewpoint of anodic performance in the fuel cell. Figure 6 shows the cell current Icell and power output P of the fuel cells as functions of the cell voltage Vcell, in which P is given by the product of Icell and Vcell. The open-circuit voltages Voc of the fuel cell equipped with the PANI/PAA-ACP and MPA-ACP electrodes were 0.70 and 0.57 V, respectively. This difference in Voc well reflects the difference in the onset potential of oxidation of the ASA resulting from ASA2P shown in Figure 5. The short-circuit currents Isc of the fuel cells equipped with the former and latter electrodes were 16.4 and 1.0 μA cm−2-anode, respectively. The Icell of the fuel cell with the PANI/PAA-ACP electrode remained almost constant in the range of Vcell from 0.4 to 0.6 V, whereas that of the cell with the MPA-ACP electrode showed no plateau. As seen from these results, the performance of the fuel cell was markedly dependent on the bioelectrochemical activity of the anode to oxidize ASA2P. The maximum power output Pmax of the fuel cell with the PANI/PAA-ACP electrode was 6.0 μW cm−2-anode, which was 70 times as large as that of the one with the MPA-ACP electrode. As for the efficiency of the electric cells, the fill factor (FF) is defined as FF=Pmax(IscVoc)−1. The values of FF were determined for the fuel cells with the PANI/PAA-ACP and MPA-ACP electrodes as 0.52 and 0.15, respectively. Thus, a good performance was achieved by the fuel cell with the PANI/PAA-ACP electrode, which demonstrates the significance of using the PANI/PAA composite for fabrication of the electrode.
However, the performance of the PANI/PAA-ACP electrode as an anode was considerably poor compared with that of the Pt-loaded carbon paper as a cathode. Figure 7 shows the effect of ASA2P concentration on the anodic current measured with the PANI/PAA-ACP electrode at 0.2 V vs Ag/AgCl. As can be seen, although the anodic current increased with increasing concentration of ASA2P, the current increase was suppressed with the concentration going up to above 2 mM. This result suggests that only a limited increase in the power output is brought about even with an increase in the concentration of the ASA2P fed to the fuel cell. In addition, the magnitude of the anodic current is relevant to the rate of dephosphorylation of ASA2P by the ACP on the PANI/PAA-ACP electrode. If the rate of dephosphorylation were too high to limit the oxidation of resulting ASA, the anodic current obtained with 10 mM ASA2P should become ca. 0.6 mA cm−2 in accordance with the result of the electrochemical oxidation of ASA with the PANI/PAA film shown in Figure 4. The performance of the PANI/PAA-ACP electrode could be improved by increasing the activity, as well as the quantity, of the immobilized ACP. The biological approach to enhance the activity of ACP is under investigation, together with the efficient method of immobilizing ACP.
Conclusions
ACP was immobilized covalently on an Au electrode bearing the PANI/PAA film prepared by electrochemical polymerization of aniline in the presence of PAA. The bioelectrochemical activity of the PANI/PAA-ACP electrode fabricated in this way was compared with that of the MPA-ACP electrode fabricated without the PANI/PAA film by immobilizing ACP on an Au electrode bearing a self-assembled monolayer formed with MPA. The PANI/PAA-ACP electrode gave a larger oxidation current at a lower potential in a ASA2P solution than those in the case of the MPA-ACP electrode. The maximum power output of 6.0 μW μcm2-anode was obtained with the ASA2P fuel cell using the PANI/PAA-ACP electrode as an anode, which was 70 times as large as that obtained with the fuel cell using the MPA-ACP electrode. The good performance of the fuel cell with the PANI/PAA-ACP electrode was attributed to the high bioelectrochemical activity of the electrode. In the present study, it was demonstrated that the PANI/PAA film brought about positive effects on the quantity of immobilized ACP, the function to scavenge ASA resulting from dephosphorylation of ASA2P by ACP and the electrochemical ability to oxidize ASA. The PANI/PAA-ACP electrode will lead to the development of the ASA2P fuel cell that is expected to be a safe and stable power source. However, the electrode still has room for improvement. As for the performance of the electrode, the activity and quantity of the immobilized ACP are the important factors. The performance could be improved by the biological approach to enhance the activity of ACP and by the efficient method of immobilizing ACP.

Covalent immobilzation of ACP on the PANI/PAA film.
References
Kamarudin, S. K., Achmad, F. & Daud, W. R. W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrog. Energy 34, 6902–6916 (2009).
Fujiwara, N., Yamazaki, S., Siroma, Z., Ioroi, T. & Yasuda, K. Direct oxidation of L-ascorbic acid on a carbon black electrode in acidic media and polymer electrolyte fuel cells. Electrochem. Commun. 8, 720–724 (2006).
Fujiwara, N., Yamazaki, S., Siroma, Z., Ioroi, T. & Yasuda, K. L-Ascorbic acid as an alternative fuel for direct oxidation fuel cells. J. Power Sources 167, 32–38 (2007).
Fujiwara, N., Yamazaki, S. & Yasuda, K. Research and development on direct polymer electrolyte fuel cells. J. Jpn. Pet. Inst. 54, 237–247 (2011).
Wu, J., Xiao, Z. Y., Ying, Y. B. & Chan, P. C. H. Development of a miniature silicon wafer fuel cell using L-ascorbic acid as fuel. J. Zhejiang. Univ.-SCI A 9, 955–960 (2008).
Peterson, R. W. & Walton, J. H. The autoxidation of ascorbic acid. J. Am. Chem. Soc. 65, 1212–1217 (1943).
Kuwahara, T., Homma, T., Kondo, M. & Shimomura, M. A novel system combining biocatalytic dephosphorylation of L-ascorbic acid 2-phosphate and electrochemical oxidation of resulting ascorbic acid. Biosens. Bioelectron 26, 3382–3385 (2011).
Austria, R., Semenzato, A. & Bettero, A. Stability of vitamin C derivatives in solution and topical formulations. J. Pharm. Biomed. Anal. 15, 795–801 (1997).
Kuwahara, T., Oshima, K., Shimomura, M. & Miyauchi, S. Immobilization of glucose oxidase and electron-mediating groups on the film of 3-methylthiophene/thiophene-3-acetic acid copolymer and its application to reagentless sensing of glucose. Polymer 36, 8091–8097 (2005).
Kuwahara, T., Homma, T., Kondo, M. & Shimomura, M. Fabrication of enzyme electrodes with a polythiophene derivative and application of them to a glucose fuel cell. Synth. Me.t 159, 1859–1864 (2009).
Liu, C., Kuwahara, T., Yamazaki, R. & Shimomura, M. Covalent immobilization of glucose oxidase on films prepared by electrochemical copolymerization of 3-methylthiophene and thiophene-3-acetic acid for amperometric sensing of glucose: effects of polymerization conditions on sensing properties. Eur. Polym. J. 43, 3264–3276 (2007).
Miyake, T., Yoshino, S., Hata, K. & Nishizawa, M. Self-regulating enzyme-nanotube ensemble films and their application as flexible electrodes for biofuel cells. J. Am. Chem. Soc. 133, 5129–5134 (2011).
Homma, T., Kondo, M., Kuwahara, T. & Shimomura, M. Electrochemical polymerization of aniline in the presence of poly(acrylic acid) and characterization of the resulting films. Polymer 53, 223–228 (2012).
Shimomura, M., Kikuchi, H., Yamauchi, T. & Miyauchi, S. Covalent immobilization of glucose oxidase on magnetite particles via graft polymerization of acrylic acid. J. Macromol. Sci. Part A-Pure Appl. Chem 33, 1687–1697 (1996).
Shimomura, M., Miyata, R., Kuwahara, T., Oshima, K. & Miyauchi, S. Immobilization of glucose oxidase on the films prepared by electrochemical copolymerization of pyrrole and 1-(2-carboxyethyl)pyrrole for glucose sensing. Eur. Polym. J. 43, 388–394 (2007).
Shimomura, M., Ohta, M., Sugiyama, N., Oshima, K., Yamauchi, T. & Miyauchi, S. Properties of α-chymotrypsin covalently immobilized on poly(acrylic acid)-grafted magnetite particles. Polym. J. 31, 274–278 (1999).
Yano, J., Hirayama, H., Harima, Y. & Kitani, A. Electrochemical and UV-visible spectroscopic study on direct oxidation of ascorbic acid on polyaniline for fuel cells. J. Electrochem. Soc. 157, B506–B511 (2010).
Ambrosi, A., Morrin, A., Smyth, M. R. & Killard, A. J. The application of conducting polymer nanoparticle electrodes to the sensing of ascorbic acid. Anal. Chim. Acta 609, 37–43 (2008).
Mondal, S. K., Raman, R. K., Shukla, A. K. & Munichandraiah, N. Electrooxidation of ascorbic acid on polyaniline and its implications to fuel cells. J. Power Sources 145, 16–20 (2005).
Takahashi, T. Determination of inorganic phosphorus and phosphocreatine, and actions of phosphoamidase and creatine phosphokinase in boar spermatozoa. SEIKAGAKU 26, 690–698 (1955).
Acknowledgements
This work was partly supported by a Grant-in-Aid for Scientific Research (B) (no. 23360302) from the Ministry of Education, Culture, Sport, Science and Technology of Japan, which is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Homma, T., Kondo, M., Kuwahara, T. et al. Immobilization of acid phosphatase on a polyaniline/poly(acrylic acid) composite film for use as the anode of a fuel cell driven with L-ascorbic acid 2-phosphate. Polym J 44, 1117–1122 (2012). https://doi.org/10.1038/pj.2012.81
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2012.81