Abstract
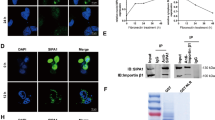
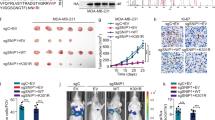
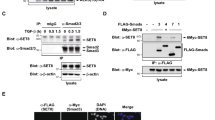
As an AEG-1/MTDH/LYRIC-binding protein, Staphylococcal nuclease domain-containing 1 (SND1) is upregulated in numerous human cancers where it has been assigned multiple functional roles. In this study, we discovered that SND1 was upregulated in breast cancer tissues, particularly the tissues from patients with distant metastases. The underlying molecular mechanisms demonstrated a novel role of SND1 in regulating the activity of transforming growth factor β1 (TGFβ1) signaling pathway, which promotes metastasis in breast cancer. We illustrated that SND1 physically associated with and recruited the histone acetylase GCN5 to the promoter regions of Smad2/3/4, and consequently enhanced the gene transcriptional activation of Smad2/3/4, which are essential downstream regulators in the TGFβ1 pathway. An electrophoretic mobility shift assay experiment further verified that SND1 could recognize the conserved domains (motifs 1 and 2) in the promoter regions of the Smad genes. Glutathione S-transferase (GST) pulldown assays indicated that the tudor domain of SND1 was responsible for the recruitment of GCN5, which increased histone H3K9 acetylation. Consistent with these results, a loss-of-function of SND1 reduced the protein level of Smads and the phosphorylation of R-Smads, thereby attenuating the R-Smad/Co-Smad depended transcription and, as a result, inhibited TGFβ signaling activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D . Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90.
Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA 2005; 102: 13909–13914.
Nam JS, Suchar AM, Kang MJ, Stuelten CH, Tang B, Michalowska AM et al. Bone sialoprotein mediates the tumor cell-targeted prometastatic activity of transforming growth factor beta in a mouse model of breast cancer. Cancer Res 2006; 66: 6327–6335.
Roberts AB, Wakefield LM . The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci USA 2003; 100: 8621–8623.
Murphy SJ, Hart SN, Halling G, Johnson SH, Smadbeck JB, Drucker T et al. Integrated genomic analysis of pancreatic ductal adenocarcinomas reveals genomic rearrangement events as significant drivers of disease. Cancer Res 2015; 76: 749–761.
Wang G, Yu Y, Sun C, Liu T, Liang T, Zhan L et al. STAT3 selectively interacts with Smad3 to antagonize TGF-beta. Oncogene 2015; 35: 4388–4398.
Yan X, Liao H, Cheng M, Shi X, Lin X, Feng XH et al. Smad7 Interacts with R-Smads to Inhibit TGF-beta/Smad Signaling. J Biol Chem 2015; 291: 382–392.
He Y, Zhang H, Yung A, Villeda SA, Jaeger PA, Olayiwola O et al. ALK5-dependent TGF-beta signaling is a major determinant of late-stage adult neurogenesis. Nat Neurosci 2014; 17: 943–952.
Yu L, Liu X, Cui K, Di Y, Xin L, Sun X et al. SND1 acts downstream of TGFbeta1 and upstream of Smurf1 to promote breast cancer metastasis. Cancer Res 2015; 75: 1275–1286.
Kuruma H, Kamata Y, Takahashi H, Igarashi K, Kimura T, Miki K et al. Staphylococcal nuclease domain-containing protein 1 as a potential tissue marker for prostate cancer. Am J Pathol 2009; 174: 2044–2050.
Wang N, Du X, Zang L, Song N, Yang T, Dong R et al. Prognostic impact of Metadherin-SND1 interaction in colon cancer. Mol Biol Rep 2012; 39: 10497–10504.
Yoo BK, Santhekadur PK, Gredler R, Chen D, Emdad L, Bhutia S et al. Increased RNA-induced silencing complex (RISC) activity contributes to hepatocellular carcinoma. Hepatology 2011; 53: 1538–1548.
Leverson JD, Koskinen PJ, Orrico FC, Rainio EM, Jalkanen KJ, Dash AB et al. Pim-1 kinase and p100 cooperate to enhance c-Myb activity. Mol Cell 1998; 2: 417–425.
Kannan N, Eaves CJ . Tipping the balance: MTDH-SND1 curbs oncogene-induced apoptosis and promotes tumorigenesis. Cell Stem Cell 2014; 15: 118–120.
Cappellari M, Bielli P, Paronetto MP, Ciccosanti F, Fimia GM, Saarikettu J et al. The transcriptional co-activator SND1 is a novel regulator of alternative splicing in prostate cancer cells. Oncogene 2014; 33: 3794–3802.
Yin J, Ding J, Huang L, Tian X, Shi X, Zhi L et al. SND1 affects proliferation of hepatocellular carcinoma cell line SMMC-7721 by regulating IGFBP3 expression. Anat Rec (Hoboken) 2013; 296: 1568–1575.
Liu X, Dong L, Zhang X, Wang B, Wang X, Li H et al. Identification of p100 target promoters by chromatin immunoprecipitation-guided ligation and selection (ChIP-GLAS). Cell Mol Immunol 2011; 8: 88–91.
Borcherding N, Kusner D, Kolb R, Xie Q, Li W, Yuan F et al. Paracrine WNT5A signaling inhibits expansion of tumor-initiating cells. Cancer Res 2015; 75: 1972–1982.
Xu J, Acharya S, Sahin O, Zhang Q, Saito Y, Yao J et al. 14-3-3zeta turns TGF-beta's function from tumor suppressor to metastasis promoter in breast cancer by contextual changes of Smad partners from p53 to Gli2. Cancer Cell 2015; 27: 177–192.
Cerami EG, Gross BE, Demir E, Rodchenkov I, Babur O, Anwar N et al. Pathway commons, a web resource for biological pathway data. Nucleic Acids Res 2011; 39: D685–D690.
Valineva T, Yang J, Palovuori R, Silvennoinen O . The transcriptional co-activator protein p100 recruits histone acetyltransferase activity to STAT6 and mediates interaction between the CREB-binding protein and STAT6. J Biol Chem 2005; 280: 14989–14996.
Su C, Zhang C, Tecle A, Fu X, He J, Song J et al. Tudor staphylococcal nuclease (Tudor-SN), a novel regulator facilitating G1/S phase transition, acting as a co-activator of E2F-1 in cell cycle regulation. J Biol Chem 2015; 290: 7208–7220.
Liu L, Jin G, Zhou X . Modeling the relationship of epigenetic modifications to transcription factor binding. Nucleic Acids Res 2015; 43: 3873–3885.
Riss A, Scheer E, Joint M, Trowitzsch S, Berger I, Tora L . Subunits of ADA-two-A-containing (ATAC) or Spt-Ada-Gcn5-acetyltrasferase (SAGA) coactivator complexes enhance the acetyltransferase activity of GCN5. J Biol Chem 2015; 290: 28997–29009.
Adams-Cioaba MA, Min J . Structure and function of histone methylation binding proteins. Biochem Cell Biol 2009; 87: 93–105.
Drabsch Y, ten Dijke P . TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev 2012; 31: 553–568.
Yang L . TGFbeta and cancer metastasis: an inflammation link. Cancer Metastasis Rev 2010; 29: 263–271.
Padua D, Massague J . Roles of TGFbeta in metastasis. Cell Res 2009; 19: 89–102.
Do TV, Kubba LA, Du H, Sturgis CD, Woodruff TK . Transforming growth factor-beta1, transforming growth factor-beta2, and transforming growth factor-beta3 enhance ovarian cancer metastatic potential by inducing a Smad3-dependent epithelial-to-mesenchymal transition. Mol Cancer Res 2008; 6: 695–705.
Li CL, Yang WZ, Chen YP, Yuan HS . Structural and functional insights into human Tudor-SN, a key component linking RNA interference and editing. Nucleic Acids Res 2008; 36: 3579–3589.
Callebaut I, Mornon JP . The human EBNA-2 coactivator p100: multidomain organization and relationship to the staphylococcal nuclease fold and to the tudor protein involved in Drosophila melanogaster development. Biochem J 1997; 321 (Pt 1): 125–132.
Quintana AM, Liu F, O'Rourke JP, Ness SA . Identification and regulation of c-Myb target genes in MCF-7 cells. BMC Cancer 2011; 11: 30.
Wang X, Liu X, Fang J, Lu Y, He J, Yao X et al. Coactivator P100 protein enhances STAT6-dependent transcriptional activation but has no effect on STAT1-mediated gene transcription. Anat Rec (Hoboken) 2010; 293: 1010–1016.
Lee SG, Kang DC, DeSalle R, Sarkar D, Fisher PB . AEG-1/MTDH/LYRIC, the beginning: initial cloning, structure, expression profile, and regulation of expression. Adv Cancer Res 2013; 120: 1–38.
Shaw N, Zhao M, Cheng C, Xu H, Saarikettu J, Li Y et al. The multifunctional human p100 protein 'hooks' methylated ligands. Nat Struct Mol Biol 2007; 14: 779–784.
Gao X, Zhao X, Zhu Y, He J, Shao J, Su C et al. Tudor staphylococcal nuclease (Tudor-SN) participates in small ribonucleoprotein (snRNP) assembly via interacting with symmetrically dimethylated Sm proteins. J Biol Chem 2012; 287: 18130–18141.
Bai X, Lu C, Jin J, Tian S, Guo Z, Chen P et al. Development of a DNA-templated peptide probe for photoaffinity labeling and enrichment of the histone modification reader proteins. Angew Chem Int Ed Engl 2016; 55: 7993–7997.
Jiang L, Smith JN, Anderson SL, Ma P, Mizzen CA, Kelleher NL . Global assessment of combinatorial post-translational modification of core histones in yeast using contemporary mass spectrometry. LYS4 trimethylation correlates with degree of acetylation on the same H3 tail. J Biol Chem 2007; 282: 27923–27934.
Vermeulen M, Eberl HC, Matarese F, Marks H, Denissov S, Butter F et al. Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell 2010; 142: 967–980.
Xie W, Mertens JC, Reiss DJ, Rimm DL, Camp RL, Haffty BG et al. Alterations of Smad signaling in human breast carcinoma are associated with poor outcome: a tissue microarray study. Cancer Res 2002; 62: 497–505.
Lee IH, Sohn M, Lim HJ, Yoon S, Oh H, Shin S et al. Ahnak functions as a tumor suppressor via modulation of TGFbeta/Smad signaling pathway. Oncogene 2014; 33: 4675–4684.
Acknowledgements
This work was supported by grant from the National Science Foundation for Distinguished Young Scholars of China (31125012 to JY), grants from the National Natural Science Foundation of China (81202102 and 81672592 to LY, 31370749 and 31670759 to JY and 81572882 to ZY), grant from Project for Innovative Research Team of Ministry of Education (IRT13085 to JY), grants from Project of Applicative Basic Research and Advanced Technology of Tianjin Municipal Science and Technology Commission (13JCQNJC12100 to LY) and grant from Specialized Research Fund for the Doctoral Program of Higher Education (20121202120018 to LY).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Yu, L., Di, Y., Xin, L. et al. SND1 acts as a novel gene transcription activator recognizing the conserved Motif domains of Smad promoters, inducing TGFβ1 response and breast cancer metastasis. Oncogene 36, 3903–3914 (2017). https://doi.org/10.1038/onc.2017.30
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.30
This article is cited by
-
The chromatin architectural regulator SND1 mediates metastasis in triple-negative breast cancer by promoting CDH1 gene methylation
Breast Cancer Research (2023)
-
ELFN1-AS1 promotes GDF15-mediated immune escape of colorectal cancer from NK cells by facilitating GCN5 and SND1 association
Discover Oncology (2023)
-
Landscape of NcRNAs involved in drug resistance of breast cancer
Clinical and Translational Oncology (2023)
-
SND1 confers chemoresistance to cisplatin-induced apoptosis by targeting GAS6-AKT in SKOV3 ovarian cancer cells
Medical Oncology (2022)
-
Long non-coding RNAs as the critical regulators of doxorubicin resistance in tumor cells
Cellular & Molecular Biology Letters (2021)