Abstract
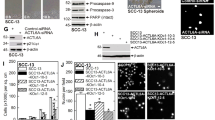
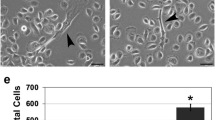
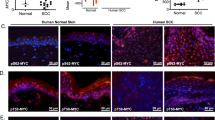
One of the key drivers for squamous cell carcinoma (SCC) proliferation is activation of the epidermal growth factor receptor (EGFR), a known proto-oncogene. However, the mechanism of EGFR-dependent SCC proliferation remains unclear. Our previous studies indicate that epidermal growth factor (EGF)-induced SCC cell proliferation requires the SH3 domain of phospholipase C-γ1 (PLC-γ1), but not its catalytic activity. The SH3 domain of PLC-γ1 is known to activate the short form of nuclear phosphatidylinositol 3-kinase enhancer (PIKE) that enhances the activity of nuclear class Ia phosphatidylinositol 3-kinase (PI3K) required for proliferation. However, PIKE has been described for more than a decade to be present exclusively in neuronal cells. In the present study, we found that PIKE was highly expressed in malignant human keratinocytes (SCC4 and SCC12B2) but had low expression in normal human keratinocytes. Immunohistochemical analysis showed strong nuclear staining of PIKE in human epidermal and tongue SCC specimens but little staining in the adjacent non-cancerous epithelium. Treatment of SCC4 cells with EGF-induced translocation of PLC-γ1 to the nucleus and binding of PLC-γ1 to the nuclear PIKE. Knockdown of PLC-γ1 or PIKE blocked EGF-induced activation of class Ia PI3K and protein kinase C-ζ and phosphorylation of nucleolin in the nucleus as well as EGF-induced SCC cell proliferation. However, inhibition of the catalytic activity of PLC-γ1 had little effect. These data suggest that PIKE has a critical role in EGF-induced SCC cell proliferation and may function as a proto-oncogene in SCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hiraishi Y, Wada T, Nakatani K, Negoro K, Fujita S . Immunohistochemical expression of EGFR and p-EGFR in oral squamous cell carcinomas. Pathol Oncol Res 2006; 12: 87–91.
Hanawa M, Suzuki S, Dobashi Y, Yamane T, Kono K, Enomoto N et al. EGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagus. Int J Cancer 2006; 118: 1173–1180.
Ettl T, Schwarz S, Kleinsasser N, Hartmann A, Reichert TE, Driemel O . Overexpression of EGFR and absence of C-KIT expression correlate with poor prognosis in salivary gland carcinomas. Histopathology 2008; 53: 567–577.
Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH . EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology 2008; 52: 738–746.
Destro A, Ceresoli GL, Falleni M, Zucali PA, Morenghi E, Bianchi P et al. EGFR overexpression in malignant pleural mesothelioma. An immunohistochemical and molecular study with clinico-pathological correlations. Lung Cancer 2006; 51: 207–215.
Schiff BA, McMurphy AB, Jasser SA, Younes MN, Doan D, Yigitbasi OG et al. Epidermal growth factor receptor (EGFR) is overexpressed in anaplastic thyroid cancer, and the EGFR inhibitor gefitinib inhibits the growth of anaplastic thyroid cancer. Clin Cancer Res 2004; 10: 8594–8602.
Livasy CA, Reading FC, Moore DT, Boggess JF, Lininger RA . EGFR expression and HER2/neu overexpression/amplification in endometrial carcinosarcoma. Gynecol Oncol 2006; 100: 101–106.
Rakosy Z, Vizkeleti L, Ecsedi S, Voko Z, Begany A, Barok M et al. EGFR gene copy number alterations in primary cutaneous malignant melanomas are associated with poor prognosis. Int J Cancer 2007; 121: 1729–1737.
Galizia G, Lieto E, Orditura M, Castellano P, Mura AL, Imperatore V et al. Epidermal growth factor receptor (EGFR) expression is associated with a worse prognosis in gastric cancer patients undergoing curative surgery. World J Surg 2007; 31: 1458–1468.
Zlobec I, Vuong T, Hayashi S, Haegert D, Tornillo L, Terracciano L et al. A simple and reproducible scoring system for EGFR in colorectal cancer: application to prognosis and prediction of response to preoperative brachytherapy. Br J Cancer 2007; 96: 793–800.
Spano JP, Lagorce C, Atlan D, Milano G, Domont J, Benamouzig R et al. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Oncol 2005; 16: 102–108.
Lurje G, Lenz HJ . EGFR signaling and drug discovery. Oncology 2009; 77: 400–410.
Ciardiello F, Tortora G . Epidermal growth factor receptor (EGFR) as a target in cancer therapy: understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur J Cancer 2003; 39: 1348–1354.
Speake G, Holloway B, Costello G . Recent developments related to the EGFR as a target for cancer chemotherapy. Curr Opin Pharmacol 2005; 5: 343–349.
Harris RC, Chuang E, Coffey RJ . EGF receptor ligands. Exp Cell Res 2003; 284: 2–13.
Wells A . EGF receptor. Int J Biochem Cell Biol 1999; 31: 637–643.
Rotin D, Honegger AM, Margolis BL, Ullrich A, Schlessinger J . Presence of SH2 domains of phospholipase C-γ1 enhances substrate phosphorylation by increasing the affinity toward the epidermal growth factor receptor. J Biol Chem 1992; 267: 9678–9683.
Kim MJ, Kim E, Ryu SH, Suh PG . The mechanism of phospholipase C-γ1 regulation. Exp Mol Med 2000; 32: 101–109.
Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J . Activation of phospholipase C γ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J 1998; 17: 414–422.
Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H et al. Protein kinase C-α activates RAF-1 by direct phosphorylation. Nature 1993; 364: 249–252.
Carroll MP, May WS . Protein kinase C-mediated serine phosphorylation directly activates Raf-1 in murine. J Biol Chem 1994; 269: 1249–1256.
Cacace AM, Ueffing M, Philipp A, Han EK, Kolch W, Weinstein IB . PKC-ɛ functions as an oncogene by enhancing activation of the Raf kinase. Oncogene 1996; 13: 2517–2526.
Zou Y, Komuro I, Yamazaki T, Aikawa R, Kudoh S, Shiojima I et al. Protein kinase C, but not tyrosine kinases or Ras, plays a critical role in angiotensin II-induced activation of Raf-1 kinase and extracellular signal-regulated protein kinases in cardiac myocytes. J Biol Chem 1996; 271: 33592–33597.
Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S . Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem 1996; 271: 23512–23519.
Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco MT, Moscat J et al. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol 1997; 17: 732–741.
Ji QS, Ermini S, Baulida J, Sun FL, Carpenter G . Epidermal growth factor signaling and mitogenesis in Plcg1 null mouse embryonic fibroblasts. Mol Biol Cell 1998; 9: 749–757.
Chen P, Xie H, Sekar MC, Gupta K, Wells A . Epidermal growth factor receptor-mediated cell motility: phospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement. J Cell Biol 1994; 127: 847–857.
Thomas SM, Coppelli FM, Wells A, Gooding WE, Song J, Kassis J et al. Epidermal growth factor receptor-stimulated activation of phospholipase C-γ1 promotes invasion of head and neck squamous cell carcinoma. Cancer Res 2003; 63: 5629–5635.
Smith MR, Liu YL, Kim SR, Bae YS, Kim CG, Kwon KS et al. PLC-γ1 Src homology domain induces mitogenesis in quiescent NIH 3T3 fibroblasts. Biochem Biophys Res Commun 1996; 222: 186–193.
Huang PS, Davis L, Huber H, Goodhart PJ, Wegrzyn RE, Oliff A et al. An SH3 domain is required for the mitogenic activity of microinjected phospholipase C-γ1. FEBS Lett 1995; 358: 287–292.
Wang Z, Gluck S, Zhang L, Moran MF . Requirement for phospholipase C-γ1 enzymatic activity in growth factor-induced mitogenesis. Mol Cell Biol 1998; 18: 590–597.
Xie Z, Chen Y, Liao EY, Jiang Y, Liu FY, Pennypacker SD . Phospholipase C-γ1 is required for the epidermal growth factor receptor-induced squamous cell carcinoma cell mitogenesis. Biochem Biophys Res Commun 2010; 397: 296–300.
Xie Z, Chen Y, Pennypacker SD, Zhou Z, Peng D . The SH3 domain, but not the catalytic domain, is required for phospholipase C-γ1 to mediate epidermal growth factor-induced mitogenesis. Biochem Biophys Res Commun 2010; 398: 719–722.
Ye K, Aghdasi B, Luo HR, Moriarity JL, Wu FY, Hong JJ et al. Phospholipase C-γ1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature 2002; 415: 541–544.
Ye K, Hurt KJ, Wu FY, Fang M, Luo HR, Hong JJ et al. PIKE: A Nuclear GTPase that Enhances PI3Kinase Activity and Is Regulated by Protein 4.1N. Cell 2000; 103: 919–930.
Toker A, Meyer M, Reddy KK, Falck JR, Aneja R, Aneja S et al. Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem 1994; 269: 32358–32367.
Carpenter G, Ji Q . Phospholipase C-γ as a signal-transducing element. Exp Cell Res 1999; 253: 15–24.
Zhou G, Seibenhener ML, Wooten MW . Nucleolin is a protein kinase C-ζ substrate. Connection between cell surface signaling and nucleus in PC12 cells. J Biol Chem 1997; 272: 31130–31137.
Ginisty H, Sicard H, Roger B, Bouvet P . Structure and function of nucleolin. J Cell Sci 1999; 112: 761–772.
Tuteja R, Tuteja N . Nucleolin: a multifunctional major nucleolar phosphoprotein. Crit Rev Eukaryot Gene Expr 1998; 33: 407–436.
Srivastava M, Pollard HB . Molecular dissection of nucleolin's role in growth and cell proliferation: new insights. FASEB J 1999; 13: 1911–1922.
Ahn J-Y, Hu Y, Kroll TG, Allard P, Ye K . PIKE-A is amplified in human cancers and prevents apoptosis by up-regulating Akt. Proc Natl Acad Sci USA 2004; 101: 6993–6998.
Liu X, Hu Y, Hao C, Rempel SA, Ye K . PIKE-A is a proto-oncogene promoting cell growth, transformation and invasion. Oncogene 2007; 26: 4918–4927.
Mizukami Y, Hirata T, Yoshida K . Nuclear translocation of PKC-ζ during ischemia and its inhibition by wortmannin, an inhibitor of phosphatidylinositol 3-kinase. FEBS Lett 1997; 401: 247–251.
Neri LM, Martelli AM, Borgatti P, Colamussi ML, Marchisio M, Capitani S . Increase in nuclear phosphatidylinositol 3-kinase activity and phosphatidylinositol (3,4,5) trisphosphate synthesis precede PKC-ζ translocation to the nucleus of NGF-treated PC12 cells. FASEB J 1999; 13: 2299–2310.
Calcerrada MC, Miguel BG, Martin L, Catalan RE, Martinez AM . Involvement of phosphatidylinositol 3-kinase in nuclear translocation of protein kinase C-ζ induced by C2-ceramide in rat hepatocytes. FEBS Lett 2002; 514: 361–365.
Tajrishi MM, Tuteja R, Tuteja N . Nucleolin: The most abundant multifunctional phosphoprotein of nucleolus. Commun Integr Biol 2011; 4: 267–275.
Storck S, Thiry M, Bouvet P . Conditional knockout of nucleolin in DT40 cells reveals the functional redundancy of its RNA-binding domains. Biol Cell 2009; 101: 153–167.
Pillai S, Bikle DD, Hincenbergs M, Elias PM . Biochemical and morphological characterization of growth and differentiation of normal human neonatal keratinocytes in a serum-free medium. J Cell Physiol 1988; 134: 229–237.
Xie Z, Bikle DD . The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-γ1 activation and human keratinocyte differentiation. J Biol Chem 2007; 282: 8695–8703.
Xie Z, Chang SM, Pennypacker SD, Liao EY, Bikle DD . Phosphatidylinositol-4-phosphate 5-kinase 1α mediates extracellular calcium-induced keratinocyte differentiation. Mol Biol Cell 2009; 20: 1695–1704.
Xie Z, Singleton PA, Bourguignon LY, Bikle DD . Calcium-induced human keratinocyte differentiation requires src- and fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-γ1. Mol Biol Cell 2005; 16: 3236–3246.
Acknowledgements
This work was supported by the grant 81072219 from the National Natural Science Foundation of China and grants 1R03DE018001 and 1R21DE019529 from the US National Institutes of Health. We thank Drs Daniel D Bikle, Chia-Ling Tu and Yongmei Wang for helpful discussions. We also thank Dr Larry L Wang (Department of Pathology, Children Hospital Los Angeles, Keck School of Medicine, University of Southern California, Los Angeles) for reviewing pathological slides.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors disclose no conflict of interest.
Rights and permissions
About this article
Cite this article
Xie, Z., Jiang, Y., Liao, EY. et al. PIKE mediates EGFR proliferative signaling in squamous cell carcinoma cells. Oncogene 31, 5090–5098 (2012). https://doi.org/10.1038/onc.2012.10
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.10