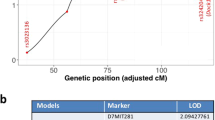
Abstract
A critical observation in sporadic cancers is that not all individuals are equally prone to developing cancer following exposure to a given environmental carcinogen. Epidemiological studies have suggested that the difference in the timing of cancer onset in response to exogenous DNA damage is likely attributable to genetic variations, such as those associated with base excision repair (BER) genes. To test this long-standing hypothesis and elucidate how a genetic variation in the BER gene flap endonuclease 1 (FEN1) results in susceptibility to environment insults and causes cancer, we established a mutant mouse model carrying a point mutation (E160D) in Fen1. We demonstrate that the E160D mutation impairs the ability of FEN1 to process DNA intermediate structures in long-patch BER using nuclear extracts or reconstituted purified BER proteins. E160D cells were more sensitive to the base-damaging agents methylnitrosourea and hydrogen peroxide, leading to DNA strand breaks, chromosomal breakage and chromosome instabilities in response these DNA insults. We further show that E160D mice are significantly more susceptible to exposure to methylnitrosourea and develop lung adenocarcinoma. Thus, our current study demonstrates that a subtle genetic variation (E160D) in BER genes (FEN1) may cause a functional deficiency in repairing base damage, such that individuals carrying the mutation or similar mutations are predisposed to chemical-induced cancer development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Asagoshi K, Liu Y, Masaoka A, Lan L, Prasad R, Horton JK et al. (2010). DNA polymerase beta-dependent long patch base excision repair in living cells. DNA Repair (Amst) 9: 109–119.
Balakrishnan L, Brandt PD, Lindsey-Boltz LA, Sancar A, Bambara RA . (2009). Long patch base excision repair proceeds via coordinated stimulation of the multienzyme DNA repair complex. J Biol Chem 284: 15158–15172.
Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S et al. (2008). γH2AX and cancer. Nat Rev Cancer 8: 957–967.
Chang JS, Wrensch MR, Hansen HM, Sison JD, Aldrich MC, Quesenberry Jr CP et al. (2009). Base excision repair genes and risk of lung cancer among San Francisco Bay Area Latinos and African-Americans. Carcinogenesis 30: 78–87.
Cistulli C, Lavrik OI, Prasad R, Hou E, Wilson SH . (2004). AP endonuclease and poly (ADP-ribose) polymerase-1 interact with the same base excision repair intermediate. DNA Repair 3: 581–591.
Demple B, Harrison L . (1994). Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem 63: 915–948.
Figueroa JD, Malats N, Real FX, Silverman D, Kogevinas M, Chanock S et al. (2007). Genetic variation in the base excision repair pathway and bladder cancer risk. Hum Genet 121: 233–242.
Frank G, Qiu J, Zheng L, Shen B . (2001). Stimulation of eukaryotic flap endonuclease-1 activities by proliferating cellnuclear antigen (PCNA) is independent of its in vitro interaction via a consensus PCNA binding region. J Biol Chem 276: 36295–36302.
Ganem NJ, Storchova Z, Pellman D . (2007). Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev 17: 157–162.
Gary R, Kim K, Cornelius HL, Park MS, Matsumoto Y . (1999). Proliferating cell nuclear antigen facilitates excision in long-patch base excision repair. J Biol Chem 274: 4354–4363.
Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T et al. (2005). Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434: 907–913.
Guo Z, Zheng L, Dai H, Zhou M, Xu H, Shen B . (2009). Human DNA polymerase β polymorphism, Arg137Gln, impairs its polymerase activity and interaction with PCNA and the cellular base excision repair capacity. Nucleic Acids Res 37: 3431–3441.
Jackson DA, Pombo A . (1998). Replicon clusters are stable units of chromosome structure: evidence that nuclear organization contributes to the efficient activation and propagation of S phase in human cells. J Cell Biol 140: 1285–1295.
Jemal A, Thomas A, Murray T, Thun M . (2002). Cancer statistics, 2002. CA Cancer J Clin 52: 23–47.
Klungland A, Lindahl T . (1997). Second pathway for completion of human DNA base excision-repair: reconstitution with purified proteins and requirement for DNase IV (FEN1). Embo J 16: 3341–3348.
Krokan HE, Standal R, Slupphaug G . (1997). DNA glycosylases in the base excision repair of DNA. Biochem J 325 (Part 1): 1–16.
Landi S, Gemignani F, Monnier S, Canzian F . (2005). A database of single-nucleotide polymorphisms and a genotyping microarray for genetic epidemiology of lung cancer. Exp Lung Res 31: 223–258.
Lengauer C, Kinzler KW, Vogelstein B . (1998). Genetic instabilities in human cancers. Nature 396: 643–649.
Lindahl T . (1993). Instability and decay of the primary structure of DNA. Nature 362: 709–715.
Lindahl T, Wood RD . (1999). Quality control by DNA repair. Science 286: 1897–1905.
Liu P, Qian L, Sung JS, de Souza-Pinto NC, Zheng L, Bogenhagen DF et al. (2008). Removal of oxidative DNA damage via FEN1-dependent long-patch base excision repair in human cell mitochondria. Mol Cell Biol 28: 4975–4987.
Liu Y, Beard WA, Shock DD, Prasad R, Hou EW, Wilson SH . (2005). DNA polymerase β and flap endonuclease 1 enzymatic specificities sustain DNA synthesis for long patch base excision repair. J Biol Chem 280: 3665–3674.
Liu Y, Kao HI, Bambara RA . (2004). Flap endonuclease 1: a central component of DNA metabolism. Annu Rev Biochem 73: 589–615.
Merrick CJ, Jackson D, Diffley JF . (2004). Visualization of altered replication dynamics after DNA damage in human cells. J Biol Chem 279: 20067–20075.
Ming Y, Huan G, Chen W, Yuefeng H, Dianke Y, Li Z et al. (2009). Functional FEN1 polymorphisms are associated with DNA damage levels and lung cancer risk. Human Mutation 30: 1320–1328.
Nakamura J, Walker VE, Upton PB, Chiang SY, Kow YW, Swenberg JA . (1998). Highly sensitive apurinic/apyrimidinic site assay can detect spontaneous and chemically induced depurination under physiological conditions. Cancer Res 58: 222–225.
Pascucci B, Russo MT, Crescenzi M, Bignami M, Dogliotti E . (2005). The accumulation of MMS-induced single strand breaks in G1 phase is recombinogenic in DNA polymerase beta defective mammalian cells. Nucleic Acids Res 33: 280–288.
Prasad R, Batra VK, Yang XP, Krahn JM, Pedersen LC, Beard WA et al. (2005). Structural insight into the DNA polymerase β deoxyribose phosphate lyase mechanism. DNA Repair (Amst) 4: 1347–1357.
Rajagopalan H, Lengauer C . (2004). Aneuploidy and cancer. Nature 432: 338–341.
Ranalli TA, Tom S, Bambara RA . (2002). AP endonuclease 1 coordinates flap endonuclease 1 and DNA ligase I activity in long patch base excision repair. J Biol Chem 277: 41715–41724.
Rotman G, Shiloh Y . (1998). ATM: from gene to function. Hum Mol Genet 7: 1555–1563.
Shen B, Singh P, Liu R, Qiu J, Zheng L, Finger LD et al. (2005). Multiple but dissectible functions of FEN-1 nucleases in nucleic acid processing, genome stability and diseases. Bioessays 27: 717–729.
Singhal RK, Wilson SH . (1993). Short gap-filling synthesis by DNA polymerase β is processive. J Biol Chem 268: 15906–15911.
Sun B, Latham KA, Dodson ML, Lloyd RS . (1995). Studies on the catalytic mechanism of five DNA glycosylases. Probing for enzyme-DNA imino intermediates. J Biol Chem 270: 19501–19508.
Vainio H, Boffetta P . (1994). Mechanisms of the combined effect of asbestos and smoking in the etiology of lung cancer. Scand J Work Environ Health 20: 235–242.
van Gent DC, Hoeijmakers JHJ, Kanaar R . (2001). Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet 2: 196–206.
Wang Y, Putnam CD, Kane MF, Zhang W, Edelmann L, Russell R et al. (2005). Mutation in Rpa1 results in defective DNA double-strand break repair, chromosomal instability and cancer in mice. Nat Genet 37: 750–755.
Wong D, Demple B . (2004). Modulation of the 5′deoxyribose-5-phosphate lyase and DNA synthesis activities of mammalian DNA polymerase β by apurinic/apyrimidinic endonuclease 1. J Biol Chem 279: 25268–25275.
Zheng L, Dai H, Zhou M, Li M, Singh P, Qiu J et al. (2007). Fen1 mutations result in autoimmunity, chronic inflammation and cancers. Nat Med 13: 812–819.
Zheng L, Zhou M, Guo Z, Lu H, Qian L, Dai H et al. (2008). Human DNA2 is a mitochondrial nuclease/helicase for efficient processing of DNA replication and repair intermediates. Mol Cell 32: 325–336.
Acknowledgements
We thank the microscopy core facility of City of Hope for technical assistance with immunofluorescence staining of MEF cells. We thank D Finger for technical advice on purification of FEN1 proteins. We thank SR da Costa and K Walker for editorial assistance. All protocols involving animal use were approved by the research animal care committee of City of Hope National Medical Center and Beckman Research Institute in compliance with the Public Health Service Policy on Use of Laboratory Animals. This work was supported by an NIH grant R01 CA073764 to BHS and a scholarship from China Scholarship Council (CSC) to HX.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Xu, H., Zheng, L., Dai, H. et al. Chemical-induced cancer incidence and underlying mechanisms in Fen1 mutant mice. Oncogene 30, 1072–1081 (2011). https://doi.org/10.1038/onc.2010.482
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.482
Keywords
This article is cited by
-
The FEN1 L209P mutation interferes with long-patch base excision repair and induces cellular transformation
Oncogene (2017)
-
Variants and haplotypes in Flap endonuclease 1 and risk of gallbladder cancer and gallstones: a population-based study in China
Scientific Reports (2015)
-
The FEN1 E359K germline mutation disrupts the FEN1–WRN interaction and FEN1 GEN activity, causing aneuploidy-associated cancers
Oncogene (2015)
-
Polyploid cells rewire DNA damage response networks to overcome replication stress-induced barriers for tumour progression
Nature Communications (2012)
-
Overexpression of androgen receptor enhances the binding of the receptor to the chromatin in prostate cancer
Oncogene (2012)