Abstract
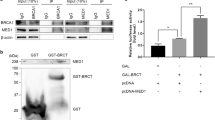
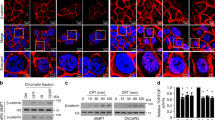
We have previously reported that the expression of antioxidative stress enzymes is upregulated by trans-hydroxytamoxifen (TOT) in breast epithelial cell lines providing protection against estrogen-induced DNA damage. This regulation involves Estrogen Receptor β (ERβ) recruitment to the Electrophile Response Element (EpRE) and a novel protein, human homolog of Xenopus gene which Prevents Mitotic Catastrophe (hPMC2). We have also demonstrated that ERβ and hPMC2 are required for TOT-dependent recruitment of poly (ADP-ribose) polymerase 1 (PARP-1) and Topoisomerase IIβ (Topo IIβ) to the EpRE. Sequence analysis reveals that the C-terminus of hPMC2 encodes a putative exonuclease domain. Using in vitro kinetic assays, we found that hPMC2 is a 3′–5′ non-processive exonuclease that degrades both single-stranded and double-stranded substrates. Mutation of two conserved carboxylate residues drastically reduced the exonuclease activity of hPMC2, indicating the relative importance of the catalytic residues. Western blot analysis of breast cancer cell lines for Quinone Reductase (QR) levels revealed that the intrinsic exonuclease activity of hPMC2 was required for TOT-induced QR upregulation. Chromatin immunoprecipitation (ChIP) assays also indicated that hPMC2 was involved in the formation of strand breaks observed with TOT treatment and is specific for the EpRE-containing region of the QR gene. We also determined that the transcription factor NF-E2-related factor-2 (Nrf2) is involved in the specificity of hPMC2 for the EpRE. In addition, we determined that the catalytic activity of hPMC2 is required for repair of abasic sites that result from estrogen-induced DNA damage. Thus, our study provides a mechanistic basis for transcriptional regulation by hPMC2 and provides novel insights into its role in cancer prevention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Abbreviations
- E2:
-
17-β estradiol
- EpRE:
-
electrophile response element
- ERE:
-
estrogen response element
- ERβ:
-
estrogen receptor β
- hPMC2:
-
human homolog of Xenopus gene which prevents mitotic catastrophe
- QR:
-
quinone reductase
- TOT:
-
trans-hydroxytamoxifen
References
Ando K, Hirao S, Kabe Y, Ogura Y, Sato I, Yamaguchi Y et al. (2008). A new APE1/Ref-1-dependent pathway leading to reduction of NF-kappaB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Res 36: 4327–4336.
Berdis AJ . (2001). Dynamics of translesion DNA synthesis catalyzed by the bacteriophage T4 exonuclease-deficient DNA polymerase. Biochemistry 40: 7180–7191.
Bianco NR, Perry G, Smith MA, Templeton DJ, Montano MM . (2003). Functional implications of antiestrogen induction of quinone reductase: inhibition of estrogen-induced deoxyribonucleic acid damage. Mol Endocrinol 17: 1344–1355.
Bolton JL, Thatcher GR . (2008). Potential mechanisms of estrogen quinone carcinogenesis. Chem Res Toxicol 21: 93–101.
Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R et al. (2006). Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta 1766: 63–78.
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM et al. (1998). Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90: 1371–1388.
Gaikwad NW, Rogan EG, Cavalieri EL . (2007). Evidence from ESI-MS for NQO1-catalyzed reduction of estrogen ortho-quinones. Free Radic Biol Med 43: 1289–1298.
Hopp TA, Weiss HL, Parra IS, Cui Y, Osborne CK, Fuqua SA . (2004). Low levels of estrogen receptor beta protein predict resistance to tamoxifen therapy in breast cancer. Clin Cancer Res 10: 7490–7499.
Hoss M, Robins P, Naven TJ, Pappin DJ, Sgouros J, Lindahl T . (1999). A human DNA editing enzyme homologous to the Escherichia coli DnaQ/MutD protein. EMBO J 18: 3868–3875.
Hsieh JC, Zinnen S, Modrich P . (1993). Kinetic mechanism of the DNA-dependent DNA polymerase activity of human immunodeficiency virus reverse transcriptase. J Biol Chem 268: 24607–24613.
Huang P . (1998). Excision of mismatched nucleotides from DNA: a potential mechanism for enhancing DNA replication fidelity by the wild-type p53 protein. Oncogene 17: 261–270.
Jordan VC . (1998). Antiestrogenic action of raloxifene and tamoxifen: today and tomorrow. J Natl Cancer Inst 90: 967–971.
Ju BG, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, Glass CK et al. (2006). A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science 312: 1798–1802.
Liehr JG . (2000). Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev 21: 40–54.
Marti TM, Fleck O . (2004). DNA repair nucleases. Cell Mol Life Sci 61: 336–354.
Mol CD, Izumi T, Mitra S, Tainer JA . (2000). DNA-bound structures and mutants reveal abasic DNA binding by APE1 and DNA repair coordination (corrected). Nature 403: 451–456.
Montano MM, Chaplin LJ, Deng H, Mesia-Vela S, Gaikwad N, Zahid M et al. (2007). Protective roles of quinone reductase and tamoxifen against estrogen-induced mammary tumorigenesis. Oncogene 26: 3587–3590.
Montano MM, Deng H, Liu M, Sun X, Singal R . (2004). Transcriptional regulation by the estrogen receptor of antioxidative stress enzymes and its functional implications. Oncogene 23: 2442–2453.
Montano MM, Jaiswal AK, Katzenellenbogen BS . (1998). Transcriptional regulation of the human quinone reductase gene by antiestrogen-liganded estrogen receptor-alpha and estrogen receptor-beta. J Biol Chem 273: 25443–25449.
Montano MM, Katzenellenbogen BS . (1997). The quinone reductase gene: a unique estrogen receptor-regulated gene that is activated by antiestrogens. Proc Natl Acad Sci USA 94: 2581–2586.
Montano MM, Wittmann BM, Bianco NR . (2000). Identification and characterization of a novel factor that regulates quinone reductase gene transcriptional activity. J Biol Chem 275: 34306–34313.
Moser MJ, Holley WR, Chatterjee A, Mian IS . (1997). The proofreading domain of Escherichia coli DNA polymerase I and other DNA and/or RNA exonuclease domains. Nucleic Acids Res 25: 5110–5118.
Nakamura T, Kuroda Y, Yamashita S, Zhang X, Miyamoto O, Tamiya T et al. (2008). Edaravone attenuates brain edema and neurologic deficits in a rat model of acute intracerebral hemorrhage. Stroke 39: 463–469.
Nakopoulou L, Lazaris AC, Panayotopoulou EG, Giannopoulou I, Givalos N, Markaki S et al. (2004). The favourable prognostic value of oestrogen receptor beta immunohistochemical expression in breast cancer. J Clin Pathol 57: 523–528.
Nguyen LH, Espert L, Mechti N, Wilson III DM . (2001). The human interferon- and estrogen-regulated ISG20/HEM45 gene product degrades single-stranded RNA and DNA in vitro. Biochemistry 40: 7174–7179.
Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD . (2003). Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J 374: 337–348.
Osborne CK, Wakeling A, Nicholson RI . (2004). Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer 90 (Suppl 1): S2–S6.
Saji S, Hirose M, Toi M . (2005). Clinical significance of estrogen receptor beta in breast cancer. Cancer Chemother Pharmacol 56 (Suppl 1): 21–26.
Skalski V, Lin ZY, Choi BY, Brown KR . (2000). Substrate specificity of the p53-associated 3′-5′ exonuclease. Oncogene 19: 3321–3329.
Sripathy SP, Chaplin LJ, Gaikwad NW, Rogan EG, Montano MM . (2008). hPMC2 is required for recruiting an ERbeta coactivator complex to mediate transcriptional upregulation of NQO1 and protection against oxidative DNA damage by tamoxifen. Oncogene 27: 6376–6384.
Wilson III DM, Takeshita M, Grollman AP, Demple B . (1995). Incision activity of human apurinic endonuclease (Ape) at abasic site analogs in DNA. J Biol Chem 270: 16002–16007.
Xanthoudakis S, Miao G, Wang F, Pan YC, Curran T . (1992). Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J 11: 3323–3335.
Yager JD, Davidson NE . (2006). Estrogen carcinogenesis in breast cancer. N Engl J Med 354: 270–282.
Acknowledgements
This work was supported by the Department of Defense Breast Cancer Postdoctoral award (BC087610) to NK and NIH Grant (CA92240) to MMM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Krishnamurthy, N., Ngam, C., Berdis, A. et al. The exonuclease activity of hPMC2 is required for transcriptional regulation of the QR gene and repair of estrogen-induced abasic sites. Oncogene 30, 4731–4739 (2011). https://doi.org/10.1038/onc.2011.186
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.186
Keywords
This article is cited by
-
A genomics approach to females with infertility and recurrent pregnancy loss
Human Genetics (2020)