Abstract
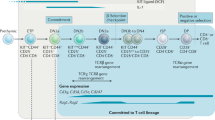
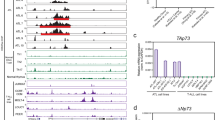
The T-cell oncogene Lim-only 2 (LMO2) critically influences both normal and malignant haematopoiesis. LMO2 is not normally expressed in T cells, yet ectopic expression is seen in the majority of T-acute lymphoblastic leukaemia (T-ALL) patients with specific translocations involving LMO2 in only a subset of these patients. Ectopic lmo2 expression in thymocytes of transgenic mice causes T-ALL, and retroviral vector integration into the LMO2 locus was implicated in the development of clonal T-cell disease in patients undergoing gene therapy. Using array-based chromatin immunoprecipitation, we now demonstrate that in contrast to B-acute lymphoblastic leukaemia, human T-ALL samples largely use promoter elements with little influence from distal enhancers. Active LMO2 promoter elements in T-ALL included a previously unrecognized third promoter, which we demonstrate to be active in cell lines, primary T-ALL patients and transgenic mice. The ETS factors ERG and FLI1 previously implicated in lmo2-dependent mouse models of T-ALL bind to the novel LMO2 promoter in human T-ALL samples, while in return LMO2 binds to blood stem/progenitor enhancers in the FLI1 and ERG gene loci. Moreover, LMO2, ERG and FLI1 all regulate the +1 enhancer of HHEX/PRH, which was recently implicated as a key mediator of early progenitor expansion in LMO2-driven T-ALL. Our data therefore suggest that a self-sustaining triad of LMO2/ERG/FLI1 stabilizes the expression of important mediators of the leukaemic phenotype such as HHEX/PRH.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Appert A, Nam CH, Lobato N, Priego E, Miguel RN, Blundell T et al. (2009). Targeting LMO2 with a peptide aptamer establishes a necessary function in overt T-cell neoplasia. Cancer Res 69: 4784–4790.
Asnafi V, Beldjord K, Libura M, Villarese P, Millien C, Ballerini P et al. (2004). Age-related phenotypic and oncogenic differences in T-cell acute lymphoblastic leukemias may reflect thymic atrophy. Blood 104: 4173–4180.
Baldus CD, Burmeister T, Martus P, Schwartz S, Gokbuget N, Bloomfield CD et al. (2006). High expression of the ETS transcription factor ERG predicts adverse outcome in acute T-lymphoblastic leukemia in adults. J Clin Oncol 24: 4714–4720.
Baldus CD, Martus P, Burmeister T, Schwartz S, Gokbuget N, Bloomfield CD et al. (2007). Low ERG and BAALC expression identifies a new subgroup of adult acute T-lymphoblastic leukemia with a highly favorable outcome. J Clin Oncol 25: 3739–3745.
Boehm T, Foroni L, Kaneko Y, Perutz MF, Rabbitts TH . (1991). The rhombotin family of cysteine-rich LIM-domain oncogenes: distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13. Proc Natl Acad Sci USA 88: 4367–4371.
Bohne A, Schlee C, Mossner M, Thibaut J, Heesch S, Thiel E et al. (2009). Epigenetic control of differential expression of specific ERG isoforms in acute T-lymphoblastic leukemia. Leuk Res 33: 817–822.
Chan WY, Follows GA, Lacaud G, Pimanda JE, Landry JR, Kinston S et al. (2007). The paralogous hematopoietic regulators Lyl1 and Scl are coregulated by Ets and GATA factors, but Lyl1 cannot rescue the early Scl−/− phenotype. Blood 109: 1908–1916.
Chiaretti S, Li X, Gentleman R, Vitale A, Vignetti M, Mandelli F et al. (2004). Gene expression profile of adult T-cell acute lymphocytic leukemia identifies distinct subsets of patients with different response to therapy and survival. Blood 103: 2771–2778.
Crable SC, Anderson KP . (2003). A PAR domain transcription factor is involved in the expression from a hematopoietic-specific promoter for the human LMO2 gene. Blood 101: 4757–4764.
Crist WM, Pui CH . (1993). Clinical implications of cytogenetic and molecular analyses of pediatric acute lymphoblastic leukemia. Stem Cells 11: 81–87.
Dave UP, Akagi K, Tripathi R, Cleveland SM, Thompson MA, Yi M et al. (2009). Murine leukemias with retroviral insertions at Lmo2 are predictive of the leukemias induced in SCID-X1 patients following retroviral gene therapy. PLoS Genet 5: e1000491.
Donaldson IJ, Chapman M, Kinston S, Landry JR, Knezevic K, Piltz S et al. (2005). Genome-wide identification of cis-regulatory sequences controlling blood and endothelial development. Hum Mol Genet 14: 595–601.
Ferrando AA, Herblot S, Palomero T, Hansen M, Hoang T, Fox EA et al. (2004). Biallelic transcriptional activation of oncogenic transcription factors in T-cell acute lymphoblastic leukemia. Blood 103: 1909–1911.
Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC et al. (2002). Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell 1: 75–87.
Follows GA, Dhami P, Gottgens B, Bruce AW, Campbell PJ, Dillon SC et al. (2006). Identifying gene regulatory elements by genomic microarray mapping of DNaseI hypersensitive sites. Genome Res 16: 1310–1319.
Garand R, Bene MC . (1993). Incidence, clinical and laboratory features, and prognostic significance of immunophenotypic subgroups in acute lymphoblastic leukemia: the GEIL experience. Recent Results Cancer Res 131: 283–295.
Gottgens B, Broccardo C, Sanchez MJ, Deveaux S, Murphy G, Gothert JR et al. (2004). The scl +18/19 stem cell enhancer is not required for hematopoiesis: identification of a 5′ bifunctional hematopoietic-endothelial enhancer bound by Fli-1 and Elf-1. Mol Cell Biol 24: 1870–1883.
Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E et al. (2008). Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest 118: 3132–3142.
Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E et al. (2003a). A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 348: 255–256.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P et al. (2003b). LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302: 415–419.
Hammond SM, Crable SC, Anderson KP . (2005). Negative regulatory elements are present in the human LMO2 oncogene and may contribute to its expression in leukemia. Leuk Res 29: 89–97.
Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H et al. (2008). Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest 118: 3143–3150.
Huang X, Brown C, Ni W, Maynard E, Rigby AC, Oettgen P . (2006). Critical role for the Ets transcription factor ELF-1 in the development of tumor angiogenesis. Blood 107: 3153–3160.
Kadrmas JL, Beckerle MC . (2004). The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol 5: 920–931.
Kruse EA, Loughran SJ, Baldwin TM, Josefsson EC, Ellis S, Watson DK et al. (2009). Dual requirement for the ETS transcription factors Fli-1 and Erg in hematopoietic stem cells and the megakaryocyte lineage. Proc Natl Acad Sci 106: 13814–13819.
Landry JR, Bonadies N, Kinston S, Knezevic K, Wilson NK, Oram SH et al. (2009). Expression of the leukemia oncogene Lmo2 is controlled by an array of tissue-specific elements dispersed over 100 kb and bound by Tal1/Lmo2, Ets, and Gata factors. Blood 113: 5783–5792.
Landry JR, Kinston S, Knezevic K, Donaldson IJ, Green AR, Gottgens B . (2005). Fli1, Elf1, and Ets1 regulate the proximal promoter of the LMO2 gene in endothelial cells. Blood 106: 2680–2687.
Larson RC, Lavenir I, Larson TA, Baer R, Warren AJ, Wadman I et al. (1996). Protein dimerization between Lmo2 (Rbtn2) and Tal1 alters thymocyte development and potentiates T cell tumorigenesis in transgenic mice. EMBO J 15: 1021–1027.
Larson RC, Osada H, Larson TA, Lavenir I, Rabbitts TH . (1995). The oncogenic LIM protein Rbtn2 causes thymic developmental aberrations that precede malignancy in transgenic mice. Oncogene 11: 853–862.
Lock RB, Liem N, Farnsworth ML, Milross CG, Xue C, Tajbakhsh M et al. (2002). The nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model of childhood acute lymphoblastic leukemia reveals intrinsic differences in biologic characteristics at diagnosis and relapse. Blood 99: 4100–4108.
MacArthur BD, Ma'ayan A, Lemischka IR . (2009). Systems biology of stem cell fate and cellular reprogramming. Nat Rev Mol Cell Biol 10: 672–681.
McCormack MP, Young LF, Vasudevan S, de Graaf CA, Codrington R, Rabbitts TH et al. (2010). The Lmo2 oncogene initiates leukemia in mice by inducing thymocyte self-renewal. Science 327: 879–883.
Noy P, Williams H, Sawasdichai A, Gaston K, Jayaraman P-S . (2010). PRH/Hhex controls cell survival through coordinate transcriptional regulation of vascular endothelial growth factor signaling. Mol Cell Biol 30: 2120–2134.
Osada H, Grutz G, Axelson H, Forster A, Rabbitts TH . (1995). Association of erythroid transcription factors: complexes involving the LIM protein RBTN2 and the zinc-finger protein GATA1. Proc Natl Acad Sci USA 92: 9585–9589.
Pimanda JE, Ottersbach K, Knezevic K, Kinston S, Chan WY, Wilson NK et al. (2007). Gata2, Fli1, and Scl form a recursively wired gene-regulatory circuit during early hematopoietic development. Proc Natl Acad Sci USA 104: 17692–17697.
Rabbitts TH, Axelson H, Forster A, Grutz G, Lavenir I, Larson R et al. (1997). Chromosomal translocations and leukaemia: a role for LMO2 in T cell acute leukaemia, in transcription and in erythropoiesis. Leukemia 11 (Suppl 3): 271–272.
Raimondi SC . (2007). T-lineage acute lymphoblastic leukemia (T-ALL). Atlas Genet Cytogenet Oncol Haematol 11: 69–81.
Royer-Pokora B, Loos U, Ludwig WD . (1991). TTG-2, a new gene encoding a cysteine-rich protein with the LIM motif, is overexpressed in acute T-cell leukaemia with the t(11;14)(p13;q11). Oncogene 6: 1887–1893.
Royer-Pokora B, Rogers M, Zhu TH, Schneider S, Loos U, Bolitz U . (1995). The TTG-2/RBTN2T cell oncogene encodes two alternative transcripts from two promoters: the distal promoter is removed by most 11p13 translocations in acute T cell leukaemia's (T-ALL). Oncogene 10: 1353–1360.
Valge-Archer V, Forster A, Rabbitts TH . (1998). The LMO1 and LDB1 proteins interact in human T cell acute leukaemia with the chromosomal translocation t(11;14)(p15;q11). Oncogene 17: 3199–3202.
van Grotel M, Meijerink JP, van Wering ER, Langerak AW, Beverloo HB, Buijs-Gladdines JG et al. (2008). Prognostic significance of molecular-cytogenetic abnormalities in pediatric T-ALL is not explained by immunophenotypic differences. Leukemia 22: 124–131.
Wadman IA, Osada H, Grutz GG, Agulnick AD, Westphal H, Forster A et al. (1997). The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. EMBO J 16: 3145–3157.
Warren AJ, Colledge WH, Carlton MB, Evans MJ, Smith AJ, Rabbitts TH . (1994). The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell 78: 45–57.
Wilson NK, Miranda-Saavedra D, Kinston S, Bonadies N, Foster SD, Calero-Nieto F et al. (2009). The transcriptional program controlled by the stem cell leukemia gene Scl/Tal1 during early embryonic hematopoietic development. Blood 113: 5456–5465.
Yamada Y, Warren AJ, Dobson C, Forster A, Pannell R, Rabbitts TH . (1998). The T cell leukemia LIM protein Lmo2 is necessary for adult mouse hematopoiesis. Proc Natl Acad Sci USA 95: 3890–3895.
Yeoh EJ, Ross ME, Shurtleff SA, Williams WK, Patel D, Mahfouz R et al. (2002). Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell 1: 133–143.
Acknowledgements
We thank the Kay Kendall Leukaemia Fund, Medical Research Council, Cancer Research UK, National Health and Research Council of Australia, the National Institute for Health Research Cambridge Biomedical Research Centre and Leukaemia and Lymphoma Research UK for grant funding. We are grateful to Michelle Hammet and Tina Hamilton for assistance with pronuclear microinjections and to Professor Sally Kinsey, Leeds Teaching Hospitals, for assistance with sourcing patient material.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Oram, S., Thoms, J., Pridans, C. et al. A previously unrecognized promoter of LMO2 forms part of a transcriptional regulatory circuit mediating LMO2 expression in a subset of T-acute lymphoblastic leukaemia patients. Oncogene 29, 5796–5808 (2010). https://doi.org/10.1038/onc.2010.320
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.320
Keywords
This article is cited by
-
Inferring gene expression from cell-free DNA fragmentation profiles
Nature Biotechnology (2022)
-
3off2: A network reconstruction algorithm based on 2-point and 3-point information statistics
BMC Bioinformatics (2016)
-
Misregulation of the proline rich homeodomain (PRH/HHEX) protein in cancer cells and its consequences for tumour growth and invasion
Cell & Bioscience (2016)
-
MAPK/ERK2 phosphorylates ERG at serine 283 in leukemic cells and promotes stem cell signatures and cell proliferation
Leukemia (2016)
-
BET protein inhibition shows efficacy against JAK2V617F-driven neoplasms
Leukemia (2014)