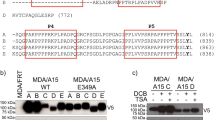
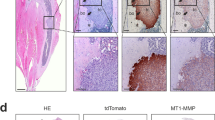
Abstract
ADAMs (a disintegrin and metalloprotease) are transmembrane proteins involved in a variety of physiological processes and tumorigenesis. Recently, ADAM8 has been associated with poor prognosis of lung cancer. However, its contribution to tumorigenesis in the context of lung cancer metastasis remains unknown. Native ADAM8 expression levels were lower in lung cancer cell lines. In contrast, we identified and characterized two novel spliced isoforms encoding truncated proteins, Δ18a and Δ14′, which were present in several tumor cell lines and not in normal cells. Overexpression of Δ18a protein resulted in enhanced invasive activity in vitro. ADAM8 and its Δ14′ isoform expression levels were markedly increased in lung cancer cells, in conditions mimicking tumor microenvironment. Moreover, addition of supernatants from Δ14′-overexpressing cells resulted in a significant increase in tartrate-resistant acid phosphatase+ cells in osteoclast cultures in vitro. These findings were associated with increased pro-osteoclastogenic cytokines interleukin (IL)-8 and IL-6 protein levels. Furthermore, lung cancer cells overexpressing Δ14′ increased prometastatic activity with a high tumor burden and increased osteolysis in a murine model of bone metastasis. Thus, the expression of truncated forms of ADAM8 by the lung cancer cells may result in the specific upregulation of their invasive and osteoclastogenic activities in the bone microenvironment. These findings suggest a novel mechanism of tumor-induced osteolysis in metastatic bone colonization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Abbreviations
- MMP:
-
metalloprotease
- NSCLC:
-
nonsmall cell lung cancer
- SCLC:
-
small cell lung cancer
- TRAP:
-
tartrate-resistant acid phosphatase
References
Amour A, Knight CG, English WR, Webster A, Slocombe PM, Knauper V et al. (2002). The enzymatic activity of ADAM8 and ADAM9 is not regulated by TIMPs. FEBS Lett 524: 154–158.
Blobel CP . (2005). ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 6: 32–43.
Cao Y, Kang Q, Zolkiewska A . (2001). Metalloprotease-disintegrin ADAM 12 interacts with alpha-actinin-1. Biochem J 357: 353–361.
Coleman RE . (1997). Skeletal complications of malignancy. Cancer 80: 1588–1594.
Choi SJ, Han JH, Roodman GD . (2001). ADAM8: a novel osteoclast stimulating factor. J Bone Miner Res 16: 814–822.
De Larco JE, Wuertz BR, Rosner KA, Erickson SA, Gamache DE, Manivel JC et al. (2001). A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol 158: 639–646.
Foley SC, Mogas AK, Olivenstein R, Fiset PO, Chakir J, Bourbeau J et al. (2007). Increased expression of ADAM33 and ADAM8 with disease progression in asthma. J Allergy Clin Immunol 119: 863–871.
Fourie AM, Coles F, Moreno V, Karlsson L . (2003). Catalytic activity of ADAM8, ADAM15, and MDC-L (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. J Biol Chem 278: 30469–30477.
Galliano MF, Huet C, Frygelius J, Polgren A, Wewer UM, Engvall E . (2000). Binding of ADAM12, a marker of skeletal muscle regeneration, to the muscle-specific actin-binding protein, alpha -actinin-2, is required for myoblast fusion. J Biol Chem 275: 13933–13939.
Garton KJ, Gough PJ, Philalay J, Wille PT, Blobel CP, Whitehead RH et al. (2003). Stimulated shedding of vascular cell adhesion molecule 1 (VCAM-1) is mediated by tumor necrosis factor-alpha-converting enzyme (ADAM 17). J Biol Chem 278: 37459–37464.
Gee JM, Knowlden JM . (2003). ADAM metalloproteases and EGFR signalling. Breast Cancer Res 5: 223–224.
Gilpin BJ, Loechel F, Mattei MG, Engvall E, Albrechtsen R, Wewer UM . (1998). A novel, secreted form of human ADAM 12 (meltrin alpha) provokes myogenesis in vivo. J Biol Chem 273: 157–166.
Gomez-Gaviro M, Dominguez-Luis M, Canchado J, Calafat J, Janssen H, Lara-Pezzi E et al. (2007). Expression and regulation of the metalloproteinase ADAM-8 during human neutrophil pathophysiological activation and its catalytic activity on L-selectin shedding. J Immunol 178: 8053–8063.
Gonzalez I, Vicent S, de Alava E, Lecanda F . (2007). EWS/FLI-1 oncoprotein subtypes impose different requirements for transformation and metastatic activity in a murine model. J Mol Med 85: 1015–1029.
Haidl ID, Huber G, Eichmann K . (2002). An ADAM family member with expression in thymic epithelial cells and related tissues. Gene 283: 163–170.
Hanahan D, Weinberg RA . (2000). The hallmarks of cancer. Cell 100: 57–70.
Higashiyama S, Nanba D . (2005). ADAM-mediated ectodomain shedding of HB-EGF in receptor cross-talk. Biochim Biophys Acta 1751: 110–117.
Hinkle CL, Diestel S, Lieberman J, Maness PF . (2006). Metalloprotease-induced ectodomain shedding of neural cell adhesion molecule (NCAM). J Neurobiol 66: 1378–1395.
Hooper NM, Lendeckel U . (2005) (eds.) The Adam Family Of Proteases. Springer: Dordrecht, 344pp.
Ishikawa N, Daigo Y, Yasui W, Inai K, Nishimura H, Tsuchiya E et al. (2004). ADAM8 as a novel serological and histochemical marker for lung cancer. Clin Cancer Res 10: 8363–8370.
Jemal A, Thomas A, Murray T, Thun M . (2002). Cancer statistics, 2002. CA Cancer J Clin 52: 23–47.
Karadag A, Zhou M, Croucher PI . (2006). ADAM-9 (MDC-9/meltrin-gamma), a member of the a disintegrin and metalloproteinase family, regulates myeloma-cell-induced interleukin-6 production in osteoblasts by direct interaction with the alpha(v)beta5 integrin. Blood 107: 3271–3278.
Kelly K, Hutchinson G, Nebenius-Oosthuizen D, Smith AJ, Bartsch JW, Horiuchi K et al. (2005). Metalloprotease-disintegrin ADAM8: expression analysis and targeted deletion in mice. Dev Dyn 232: 221–231.
Kim T, Oh J, Woo JM, Choi E, Im SH, Yoo YJ et al. (2006). Expression and relationship of male reproductive ADAMs in mouse. Biol Reprod 74: 744–750.
King NE, Zimmermann N, Pope SM, Fulkerson PC, Nikolaidis NM, Mishra A et al. (2004). Expression and regulation of a disintegrin and metalloproteinase (ADAM) 8 in experimental asthma. Am J Respir Cell Mol Biol 31: 257–265.
Kveiborg M, Albrechtsen R, Couchman JR, Wewer UM . (2008). Cellular roles of ADAM12 in health and disease. Int J Biochem Cell Biol 40: 1685–1702.
Lu X, Lu D, Scully MF, Kakkar VV . (2007). Structure-activity relationship studies on ADAM protein-integrin interactions. Cardiovasc Hematol Agents Med Chem 5: 29–42.
Mainiero F, Soriani A, Strippoli R, Jacobelli J, Gismondi A, Piccoli M et al. (2000). RAC1/P38 MAPK signaling pathway controls beta1 integrin-induced interleukin-8 production in human natural killer cells. Immunity 12: 7–16.
Mandelin J, Li TF, Hukkanen MV, Liljestrom M, Chen ZK, Santavirta S et al. (2003). Increased expression of a novel osteoclast-stimulating factor, ADAM8, in interface tissue around loosened hip prostheses. J Rheumatol 30: 2033–2038.
Matsuno O, Miyazaki E, Nureki S, Ueno T, Kumamoto T, Higuchi Y . (2006). Role of ADAM8 in experimental asthma. Immunol Lett 102: 67–73.
Matsuno O, Miyazaki E, Nureki S, Ueno T, Ando M, Ito K et al. (2007). Elevated soluble ADAM8 in bronchoalveolar lavage fluid in patients with eosinophilic pneumonia. Int Arch Allergy Immunol 142: 285–290.
Mundy GR . (2002). Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2: 584–593.
Naus S, Richter M, Wildeboer D, Moss M, Schachner M, Bartsch JW . (2004). Ectodomain shedding of the neural recognition molecule CHL1 by the metalloprotease-disintegrin ADAM8 promotes neurite outgrowth and suppresses neuronal cell death. J Biol Chem 279: 16083–16090.
Naus S, Reipschlager S, Wildeboer D, Lichtenthaler SF, Mitterreiter S, Guan Z et al. (2006). Identification of candidate substrates for ectodomain shedding by the metalloprotease-disintegrin ADAM8. Biol Chem 387: 337–346.
Ohtsu H, Dempsey PJ, Eguchi S . (2006). ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol 291: C1–10.
Ohtsuka T, Shiomi T, Shimoda M, Kodama T, Amour A, Murphy G et al. (2006). ADAM28 is overexpressed in human non-small cell lung carcinomas and correlates with cell proliferation and lymph node metastasis. Int J Cancer 118: 263–273.
Rao H, Lu G, Kajiya H, Garcia-Palacios V, Kurihara N, Anderson J et al. (2006). Alpha9beta1: a novel osteoclast integrin that regulates osteoclast formation and function. J Bone Miner Res 21: 1657–1665.
Reiss K, Ludwig A, Saftig P . (2006). Breaking up the tie: disintegrin-like metalloproteinases as regulators of cell migration in inflammation and invasion. Pharmacol Ther 111: 985–1006.
Roemer A, Schwettmann L, Jung M, Roigas J, Kristiansen G, Schnorr D et al. (2004a). Increased mRNA expression of ADAMs in renal cell carcinoma and their association with clinical outcome. Oncol Rep 11: 529–536.
Roemer A, Schwettmann L, Jung M, Stephan C, Roigas J, Kristiansen G et al. (2004b). The membrane proteases ADAMS and hepsin are differentially expressed in renal cell carcinoma Are they potential tumor markers? J Urol 172: 2162–2166.
Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J et al. (2004). Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol 164: 769–779.
Schlomann U, Wildeboer D, Webster A, Antropova O, Zeuschner D, Knight CG et al. (2002). The metalloprotease disintegrin ADAM8. Processing by autocatalysis is required for proteolytic activity and cell adhesion. J Biol Chem 277: 48210–48219.
Seals DF, Courtneidge SA . (2003). The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev 17: 7–30.
Stautz D, Sanjay A, Hansen MT, Albrechtsen R, Wewer U, Kveiborg M . (2010). ADAM12 localizes with c-Src to actin-rich structures at the cell periphery and regulates Src kinase activity. Exp Cell Res 316: 55–67.
Takeda S, Igarashi T, Mori H . (2007). Crystal structure of RVV-X: an example of evolutionary gain of specificity by ADAM proteinases. FEBS Lett 581: 5859–5864.
Valkovskaya N, Kayed H, Felix K, Hartmann D, Giese NA, Osinsky SP et al. (2007). ADAM8 expression is associated with increased invasiveness and reduced patient survival in pancreatic cancer. J Cell Mol Med 11: 1162–1174.
Verrier S, Hogan A, McKie N, Horton M . (2004). ADAM gene expression and regulation during human osteoclast formation. Bone 35: 34–46.
Vicent S, Luis-Ravelo D, Anton I, Garcia-Tunon I, Borras-Cuesta F, Dotor J et al. (2008). A novel lung cancer signature mediates metastatic bone colonization by a dual mechanism. Cancer Res 68: 2275–2285.
Wildeboer D, Naus S, Amy Sang QX, Bartsch JW, Pagenstecher A . (2006). Metalloproteinase disintegrins ADAM8 and ADAM19 are highly regulated in human primary brain tumors and their expression levels and activities are associated with invasiveness. J Neuropathol Exp Neurol 65: 516–527.
Yoshida S, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S . (1990). Molecular cloning of cDNA encoding MS2 antigen, a novel cell surface antigen strongly expressed in murine monocytic lineage. Int Immunol 2: 585–591.
Acknowledgements
We thank especially S Martínez and the members of the Morphology and Animal Core Facilities. C Berasain for critical reading of the article. This work was supported by ‘UTE project FIMA’ agreement, and RTICCC C03/10, FIT-090100-2005-46, PI042284, PI070031 and SAF-2009-11280 (to FL). FL is also supported by funds from the I3 Program, ‘La Caixa Foundation’, and is a recipient of the ‘Ortiz de Landázuri’ award (67/2005, Government of Navarra).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Hernández, I., Moreno, J., Zandueta, C. et al. Novel alternatively spliced ADAM8 isoforms contribute to the aggressive bone metastatic phenotype of lung cancer. Oncogene 29, 3758–3769 (2010). https://doi.org/10.1038/onc.2010.130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.130
Keywords
This article is cited by
-
USP15 negatively regulates lung cancer progression through the TRAF6-BECN1 signaling axis for autophagy induction
Cell Death & Disease (2022)
-
Bone metastases
Nature Reviews Disease Primers (2020)
-
microRNA-590 suppresses the tumorigenesis and invasiveness of non-small cell lung cancer cells by targeting ADAM9
Molecular and Cellular Biochemistry (2016)
-
ADAM8 as a drug target in pancreatic cancer
Nature Communications (2015)
-
Expression of A disintegrin and metalloprotease 8 is associated with cell growth and poor survival in colorectal cancer
BMC Cancer (2014)