Key Points
-
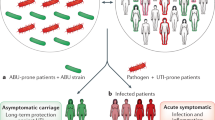
The NLRP3 inflammasome is activated during infection with uropathogenic Escherichia coli (UPEC) in an α-haemolysin-dependent manner, and exaggerated activation of this inflammasome can cause symptomatic infection
-
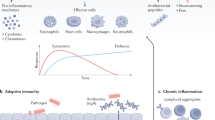
Inflammasome-dependent pyroptosis promotes exfoliation of UPEC-infected urothelial cells, thereby resolving the infection and reducing the incidence of chronic cystitis; however, pyroptosis might also increase latency by permitting microbial access to deeper urothelial layers
-
Autophagy dampens IL-1β-release and endorses UPEC latency by promoting the establishment of quiescent intracellular reservoirs (QIRs), which are reduced in cells in which autophagy is defective
-
Group B Streptococcus activates the NLRP3 inflammasome, which is dependent on β-haemolysin-mediated lysosomal leakage and RNA escape; however, the mechanistic details remain unclear
-
IL-1β levels are markedly enhanced during acute pyelonephritis, and could serve as a diagnostic marker for acute pyelonephritis
-
Inflammasome activation and IL-1β secretion have important roles in various murine models of bladder inflammation, representing viable pharmaceutical targets
Abstract
Urinary tract infections (UTIs) cause a huge burden of morbidity worldwide with recurrent UTIs becoming increasingly frequent owing to the emergence of antibiotic-resistant bacterial strains. Interactions between the innate and adaptive immune responses to pathogens colonizing the urinary tract have been the focus of much research. Inflammasomes are part of the innate immune defence and can respond rapidly to infectious insult. Assembly of the multiprotein inflammasome complex activates caspase-1, processes proinflammatory cytokines IL-1β and IL-18, and induces pyroptosis. These effector pathways, in turn, act at different levels to either prevent or resolve infection, or eliminate the infectious agent itself. In certain instances, inflammasome activation promotes tissue pathology; however, the precise functions of inflammasomes in UTIs remain unexplored. An improved understanding of inflammasomes could provide novel approaches for the design of diagnostics and therapeutics for complicated UTIs, enabling us to overcome the challenge of drug resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Foxman, B. & Frerichs, R. R. Epidemiology of urinary tract infection: I. Diaphragm use and sexual intercourse. Am. J. Public Health 75, 1308–1313 (1985).
Flores-Mireles, A. L., Walker, J. N., Caparon, M. & Hultgren, S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284 (2015).
Hanna-Wakim, R. H. et al. Epidemiology and characteristics of urinary tract infections in children and adolescents. Front. Cell. Infect. Microbiol. 5, 45 (2015).
Foxman, B., Barlow, R., D'Arcy, H., Gillespie, B. & Sobel, J. D. Urinary tract infection: self-reported incidence and associated costs. Ann. Epidemiol. 10, 509–515 (2000).
Dielubanza, E. J. & Schaeffer, A. J. Urinary tract infections in women. Med. Clin. North Am. 95, 27–41 (2011).
Dwyer, P. L. & O'Reilly, M. Recurrent urinary tract infection in the female. Curr. Opin. Obstet. Gynecol. 14, 537–543 (2002).
Geerlings, S. E., Beerepoot, M. A. J. & Prins, J. M. Prevention of recurrent urinary tract infections in women. Antimicrobial and nonantimicrobial strategies. Infect. Dis. Clin. North Am. 28, 135–147 (2014).
Stamm, W. E. & Norrby, S. R. Urinary tract infections: disease panorama and challenges. J. Infect. Dis. 183 (Suppl. 1), S1–S4 (2001).
Fleming, E., Heil, E. L. & Hynicka, L. M. Treatment strategy for a multidrug-resistant Klebsiella UTI. Ann. Pharmacother. 48, 123–127 (2014).
Colodner, R., Kometiani, I., Chazan, B. & Raz, R. Risk factors for community-acquired urinary tract infection due to quinolone-resistant E. coli. Infection 36, 41–45 (2008).
Paschke, A. A., Zaoutis, T., Conway, P. H., Xie, D., Keren, R. Previous antimicrobial exposure is associated with drug-resistant urinary tract infections in children. Pediatrics 125, 664–672 (2010).
Gupta, K., Hooton, T. M. & Stamm, W. E. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann. Intern. Med. 135, 41–50 (2001).
Gupta, K., Sahm, D. F., Mayfield, D. & Stamm, W. E. Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: a nationwide analysis. Clin. Infect. Dis. 33, 89–94 (2001).
Foxman, B. Urinary tract infection syndromes. Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. North Am. 28, 1–13 (2014).
Levison, M. E. & Kaye, D. Treatment of complicated urinary tract infections with an emphasis on drug-resistant gram-negative uropathogens. Curr. Infect. Dis. Rep. 15, 109–115 (2013).
Ragnarsdottir, B., Lutay, N., Gronberg-Hernandez, J., Koves, B. & Svanborg, C. Genetics of innate immunity and UTI susceptibility. Nat. Rev. Urol. 8, 449–468 (2011).
Spencer, J. D., Schwaderer, A. L., Becknell, B., Watson, J. & Hains, D. S. The innate immune response during urinary tract infection and pyelonefritis. Pediatr. Nephrol. 29, 1139–1149 (2015).
Godaly, G., Ambite, I. & Svanborg, C. Innate immunity and genetic determinants of urinary tract infection susceptibility. Curr. Opin. Infect. Dis. 28, 88–96 (2015).
Creagh, E. M. & O'Neill, L. A. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 27, 352–357 (2006).
Anand, P. K. et al. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488, 389–393 (2012).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145 (2001).
Kummer, J. A. et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J. Histochem. Cytochem. 55, 443–452 (2007).
Hughes, F. M. et al. Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation. Am. J. Physiol. Renal Physiol. 306, F299–F308 (2014).
Hughes, F. M., Turner, D. P. & Todd Purves, J. The potential repertoire of the innate immune system in the bladder: expression of pattern recognition receptors in the rat bladder and a rat urothelial cell line (MYP3 cells). Int. Urol. Nephrol. 47, 1953–1964 (2015).
Ting, J. P. Y. et al. The NLR gene family: an official nomenclature. Immunity 28, 285–287 (2008).
Bürckstümmer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272 (2009).
Jin, T., Perry, A., Smith, P., Jiang, J. & Xiao, T. S. Structure of the absent in melanoma 2 (AIM2) pyrin domain provides insights into the mechanisms of AIM2 autoinhibition and inflammasome assembly. J. Biol. Chem. 288, 13225–13235 (2013).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Rathinam, V. A. K. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Jin, T. et al. Structures of the HIN domain: DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 36, 561–571 (2012).
Khare, S. et al. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity 36, 464–476 (2012).
Wlodarska, M. et al. NLRP6 inflammasome orchestrates the colonic host–microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059 (2014).
Vladimer, G. I. et al. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity 37, 96–107 (2012).
Minkiewicz, J., de Rivero Vaccari, J. P. & Keane, R. W. Human astrocytes express a novel NLRP2 inflammasome. Glia 61, 1113–1121 (2013).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Anand, P. K., Malireddi, R. K. S. & Kanneganti, T. D. Role of the Nlrp3 inflammasome in microbial infection. Front. Microbiol. 2, 12 (2011).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002).
Guo, H., Callaway, J. B. & Ting, J. P.-Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 21, 677–687 (2015). A recent review covering the activation mechanisms of inflammasomes and their role in inflammatory diseases.
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Bergsbaken, T., Fink, S. L. & Cookson, B. T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 (2009).
Fink, S. L. & Cookson, B. T. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 73, 1907–1916 (2005).
Gurung, P. et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 192, 1835–1846 (2014).
Man, S. M. et al. Salmonella infection induces recruitment of caspase-8 to the inflammasome to modulate IL-1β production. J. Immunol. 191, 5239–5246 (2013).
Monie, T. P. & Bryant, C. E. Caspase-8 functions as a key mediator of inflammation and pro-IL-1β processing via both canonical and non-canonical pathways. Immunol. Rev. 265, 181–193 (2015).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Brodsky, I. E. & Medzhitov, R. Pyroptosis: macrophage suicide exposes hidden invaders. Curr. Biol. 21, R72–R75 (2011).
He, Y. & Amer, A. O. Microbial modulation of host apoptosis and pyroptosis. Front. Cell. Infect. Microbiol. 4, 1–3 (2014).
Martinon, F., Pétrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Netea, M. G. et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood 113, 2324–2335 (2009).
Bauernfeind, F. G. et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Gurung, P. et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF)-mediated caspase-11 protease production integrates toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J. Biol. Chem. 287, 34474–34483 (2012).
Rathinam, V. A. K. et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150, 606–619 (2012).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Vigano, E. et al. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat. Commun. 6, 1–13 (2015).
Casson, C. N. et al. Human caspase-4 mediates noncanonical inflammasome activation against gram-negative bacterial pathogens. Proc. Natl Acad. Sci. USA 112, 6688–6693 (2015).
Muñoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Pétrilli, V. et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 (2007).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Lupfer, C. et al. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat. Immunol. 14, 480–488 (2013).
Lupfer, C. R. et al. Reactive oxygen species regulate caspase-11 expression and activation of the non-canonical NLRP3 inflammasome during enteric pathogen infection. PLoS Pathog. 10, e1004410 (2014).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Iyer, S. et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39, 311–323 (2013).
Kuemmerle-Deschner, J. B. CAPS — pathogenesis, presentation and treatment of an autoinflammatory disease. Semin. Immunopathol. 37, 377–385 (2015).
Vandanmagsar, B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 (2011).
Stienstra, R. et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc. Natl Acad. Sci. USA 108, 15324–15329 (2011).
Vande Walle, L. et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 512, 69–73 (2014).
Abraham, S. N. et al. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae\Identification and characterization of E. coli type-1 pilus tip adhesion protein. Nature 336, 682–684 (1988).
Martinez, J. J., Mulvey, M. A., Schilling, J. D., Pinkner, J. S. & Hultgren, S. J. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19, 2803–2812 (2000).
Fukushi, Y., Orikasa, S. & Kagayama, M. An electron microscopic study of the interaction between vesical epitherlium and E. Coli. Invest. Urol. 17, 61–68 (1979).
Mulvey, M. A. et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282, 1494–1497 (1998).
Song, J. et al. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc. Natl Acad. Sci. USA 106, 14966–14971 (2009).
Anderson, G. G. et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301, 105–107 (2003).
Anderson, G. G., Dodson, K. W., Hooton, T. M. & Hultgren, S. J. Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol. 12, 424–430 (2004).
Mysorekar, I. U. & Hultgren, S. J. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl Acad. Sci. USA 103, 14170–14175 (2006).
Abraham, S. N. & Miao, Y. The nature of immune responses to urinary tract infections. Nat. Rev. Immunol. 15, 655–663 (2015). A recent review on the current knowledge of innate immune responses to UTIs.
Schilling, J. D., Martin, S. M., Hung, C. S., Lorenz, R. G. & Hultgren, S. J. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc. Natl Acad. Sci. USA 100, 4203–4208 (2003).
Schilling, J. D., Mulvey, M. A., Vincent, C. D., Lorenz, R. G. & Hultgren, S. J. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J. Immunol. 166, 1148–1155 (2001).
Hagberg, L. et al. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect. Immun. 46, 839–844 (1984).
Andersen-Nissen, E. et al. Cutting edge: Tlr5−/− mice are more susceptible to Escherichia coli urinary tract infection. J. Immunol. 178, 4717–4720 (2007).
Zhang, D. et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303, 1522–1526 (2004).
Frendéus, B. et al. Interleukin 8 receptor deficiency confers susceptibility to acute experimental pyelonephritis and may have a human counterpart. J. Exp. Med. 192, 881–890 (2000).
Yin, X. et al. Association of Toll-like receptor 4 gene polymorphism and expression with urinary tract infection types in adults. PLoS ONE 5, e14223 (2010).
Hawn, T. R. et al. Genetic variation of the human urinary tract innate immune response and asymptomatic bacteriuria in women. PLoS ONE 4, e8300 (2009).
Zhang, X. et al. Enterohemorrhagic Escherichia coli specific enterohemolysin induced IL-1beta in human macrophages and EHEC-induced IL-1beta required activation of NLRP3 inflammasome. PLoS ONE 7, e50288 (2012).
Schaale, K. et al. Strain- and host species-specific inflammasome activation, IL-1β release, and cell death in macrophages infected with uropathogenic Escherichia coli. Mucosal Immunol. 9, 124–136 (2015). This paper demonstrates the activation of the inflammasome and cell death by uropathogenic E. coli.
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
Mabbett, A. N. et al. Virulence properties of asymptomatic bacteriuria Escherichia coli. Int. J. Med. Microbiol. 299, 53–63 (2009).
Smith, Y. C., Rasmussen, S. B., Grande, K. K., Conran, R. M. & O'Brien, A. D. Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intraurethral inoculation of mice. Infect. Immun. 76, 2978–2990 (2008).
Waldhuber, A., Puthia, M. & Wieser, A. Uropathogenic Escherichia coli strain CFT073 disrupts NLRP3 inflammasome activation. J. Clin. Invest. 126, 2425–2436 (2016). A recent paper revealing the inhibition of the NLRP3 inflammasome by a UPEC virulence factor, TcpC.
Schubert, S. et al. Prevalence and phylogenetic history of the TcpC virulence determinant in Escherichia coli. Int. J. Med. Microbiol. 300, 429–434 (2010).
Cirl, C. et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat. Med. 14, 399–406 (2008).
Nagamatsu, K. et al. Dysregulation of Escherichia coli α-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc. Natl Acad. Sci. USA 112, E871–E880 (2015). This paper highlights the activation of caspase-1-dependent pyroptosis by UPEC virulence factor, α-haemolysin. Additionally, it demonstrates increased urothelium exfoliation and reduced murine bladder infection upon α-haemolysin overexpression.
Mulvey, M. A., Schilling, J. D., Martinez, J. J. & Hultgren, S. J. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl Acad. Sci. USA 97, 8829–8835 (2000).
Dhakal, B. K. & Mulvey, M. A. The UPEC pore-forming toxin α-hemolysin triggers proteolysis of host proteins to disrupt cell adhesion, inflammatory, and survival pathways. Cell Host Microbe 11, 58–69 (2012).
Mulvey, M. A., Schilling, J. D. & Hultgren, S. J. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69, 4572–4579 (2001).
Schwartz, D. J., Conover, M. S., Hannan, T. J. & Hultgren, S. J. Uropathogenic Escherichia coli superinfection enhances the severity of mouse bladder infection. PLoS Pathog. 11, 1–12 (2015). This paper reveals the importance of reintroduction of bacteria (superinfection) and the possible involvement of caspases in the development of recurrent infection following acute UTI.
Klumpp, D. J. et al. Uropathogenic Escherichia coli induces extrinsic and intrinsic cascades to initiate urothelial apoptosis. Infect. Immun. 74, 5106–5113 (2006).
Levine, B., Mizushima, N. & Virgin, H. W. Autophagy in immunity and inflammation. Nature 469, 323–335 (2011).
Deretic, V., Saitoh, T. & Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 13, 722–737 (2013).
Kuballa, P., Nolte, W. M., Castoreno, A. B. & Xavier, R. J. Autophagy and the immune system. Annu. Rev. Immunol. 30, 611–646 (2012).
Anand, P. K. et al. TLR2 and RIP2 pathways mediate autophagy of Listeria monocytogenes via extracellular signal-regulated kinase (ERK) activation. J. Biol. Chem. 286, 42981–42991 (2011).
Feng, Y., He, D., Yao, Z. & Klionsky, D. J. The machinery of macroautophagy. Cell Res. 24, 24–41 (2014).
Ryter, S. W. & Choi, A. M. K. Autophagy: an integral component of the mammalian stress response. J. Biochem. Pharmacol. Res. 1, 176–188 (2013).
Plantinga, T. S., Joosten, L. A. B. & Netea, M. G. ATG16L1 polymorphisms are associated with NOD2-induced hyperinflammation. Autophagy 7, 1074–1075 (2011).
Hampe, J. et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 39, 207–211 (2007).
Saitoh, T. et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1 beta production. Nature 456, 264–268 (2008).
Symington, J. W. et al. ATG16L1 deficiency in macrophages drives clearance of uropathogenic E. coli in an IL-1β-dependent manner. Mucosal Immunol. 8, 1–12 (2015). This paper investigates the role of autophagy in UPEC infection, and reveals protection against UPEC in the absence of autophagic protein ATG16L1.
Wang, C. et al. Atg16L1 deficiency confers protection from uropathogenic Escherichia coli infection in vivo. Proc. Natl Acad. Sci. USA 109, 11008–11013 (2012).
Shi, C.-S. et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 13, 255–263 (2012).
Harris, J. et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J. Biol. Chem. 286, 9587–9597 (2011).
Lassen, K. G. et al. Atg16L1 T300A variant decreases selective autophagy resulting in altered cytokine signaling and decreased antibacterial defense. Proc. Natl Acad. Sci. USA 111, 7741–7746 (2014).
Schuchat, A. Group B streptococcus. Lancet 353, 51–56 (1999).
Skoff, T. H. et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin. Infect. Dis. 49, 85–92 (2009).
Ulett, G. C., Maclean, K. H., Nekkalapu, S., Cleveland, J. L. & Adderson, E. E. Mechanisms of group B streptococcal-induced apoptosis of murine macrophages. J. Immunol. 175, 2555–2562 (2005).
Ulett, G. C. et al. Group B Streptococcus (GBS) urinary tract infection involves binding of GBS to bladder uroepithelium and potent but GBS-specific induction of interleukin 1alpha. J. Infect. Dis. 201, 866–870 (2010). This paper examines the production of cytokines IL-1α and IL-1β following attachment of Group B Streptococcus to the bladder epithelial cells.
Zaleznik, D. F. et al. Invasive disease due to group B Streptococcus in pregnant women and neonates from diverse population groups. Clin. Infect. Dis. 30, 276–281 (2000).
Muñoz, P. et al. Group B Streptococcus: a cause of urinary tract infection in nonpregnant adults. Clin. Infect. Dis. 14, 492–496 (1992).
Falagas, M. E., Rosmarakis, E. S., Avramopoulos, I. & Vakalis, N. Streptococcus agalactiae infections in non-pregnant adults: single center experience of a growing clinical problem. Med. Sci. Monit. 12, CR447–CR451 (2006).
Williams, P. A. et al. Production of tumor necrosis factor by human cells in vitro and in vivo, induced by group B streptococci. J. Pediatr. 123, 292–300 (1993).
Mancuso, G. et al. Type I IFN signaling is crucial for host resistance against different species of pathogenic bacteria. J. Immunol. 178, 3126–3133 (2007).
Gross, O. et al. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 36, 388–400 (2012).
Billips, B. K. et al. Modulation of host innate immune response in the bladder by uropathogenic Escherichia coli. Infect. Immun. 75, 5353–5360 (2007).
Costa, A. et al. Activation of the NLRP3 inflammasome by group B streptococci. J. Immunol. 188, 1953–1960 (2012). This paper investigates the activation of the NLRP3 inflammasome by Group B Streptroccocus via its virulence factor β-haemolysin.
Biondo, C. et al. The interleukin-1β/CXCL1/2/neutrophil axis mediates host protection against group B streptococcal infection. Infect. Immun. 82, 4508–4517 (2014).
Charrel-Dennis, M. et al. TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell Host Microbe 4, 543–554 (2008).
Gupta, R. et al. RNA and beta;-hemolysin of group B streptococcus induce interleukin-1β (IL-1β) by activating NLRP3 inflammasomes in mouse macrophages. J. Biol. Chem. 289, 13701–13705 (2014).
Li, J. et al. DDX19A senses viral RNA and mediates NLRP3-dependent inflammasome activation. J. Immunol. 195, 5732–5749 (2015).
Mitoma, H. et al. The DHX33 RNA helicase senses cytosolic RNA and activates the NLRP3 inflammasome. Immunity 39, 123–135 (2013).
Amna, M. A., Chazan, B., Raz, R., Edelstein, H. & Colodner, R. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection 41, 473–477 (2013).
Melzer, M. & Welch, C. Outcomes in UK patients with hospital-acquired bacteraemia and the risk of catheter-associated urinary tract infections. Postgrad. Med. J. 89, 329–334 (2013).
Mittal, R., Aggarwal, S., Sharma, S., Chhibber, S. & Harjai, K. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J. Infect. Public Health 2, 101–111 (2009).
Mah, T. F. et al. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426, 306–310 (2003).
Mah, T. F. C. & O'Toole, G. A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9, 34–39 (2001).
Costerton, J. W., Stewart, P. S. & Greenberg, E. P. Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322 (1999).
Poyet, J. L. et al. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 276, 28309–28313 (2001).
Molofsky, A. B. et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J. Cell Biol. 173, 1093–1104 (2006).
Ren, T., Zamboni, D. S., Roy, C. R., Dietrich, W. F. & Vance, R. E. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2, e18 (2006).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat. Immunol. 7, 576–582 (2006).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin-1β via Ipaf. Nat. Immunol. 7, 569–575 (2006).
Zamboni, D. S. et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat. Immunol. 7, 318–325 (2006).
Suzuki, T. et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 3, 1082–1091 (2007).
Franchi, L. et al. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur. J. Immunol. 37, 3030–3039 (2007).
Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600 (2011).
Kofoed, E. M. & Vance, R. E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477, 592–595 (2011).
Lightfield, K. L. et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat. Immunol. 9, 1171–1178 (2008).
Miao, E. A. et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl Acad. Sci. USA 107, 3076–3080 (2010).
Sutterwala, F. S. et al. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 204, 3235–3245 (2007).
Lindestam Arlehamn, C. S. & Evans, T. J. Pseudomonas aeruginosa pilin activates the inflammasome. Cell. Microbiol. 13, 388–401 (2011).
Cohen, T. S. & Prince, A. S. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J. Clin. Invest. 123, 1630–1637 (2013).
Schultz, M. J. et al. Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L285–L290 (2002).
Mittal, R., Sharma, S., Chhibber, S. & Harjai, K. Evaluation of tumour necrosis factor-alpha and interleukin-1beta in an experimental pyelonephritis model induced with planktonic and biofilms cells of Pseudomonas aeruginosa. Can. J. Infect. Dis. Med. Microbiol. 20, 35–42 (2009). This paper suggests a role for neutrophils in P. aeruginosa -induced pyelonephritis.
Sabharwal, N., Chhibber, S. & Harjai, K. New possibility for providing protection against urinary tract infection caused by Pseudomonas aeruginosa by non-adjuvanted flagellin 'b' induced immunity. Immunol. Lett. 162, 229–238 (2014).
Bhat, R. G., Katy, T. A. & Place, F. C. Pediatric urinary tract infections. Emerg. Med. Clin. North Am. 29, 637–653 (2011).
Lin, K. Y. et al. Acute pyelonephritis and sequelae of renal scar in pediatric first febrile urinary tract infection. Pediatr. Nephrol. 18, 362–365 (2003).
Colgan, R., Williams, M. & Johnson, J. R. Diagnosis and treatment of acute pyelonephritis in women. Am. Fam. Physician 84, 519–526 (2011).
Sheu, J.-N. et al. Urine interleukin-1beta in children with acute pyelonephritis and renal scarring. Nephrology (Carlton). 12, 487–493 (2007). This paper examines the correlation between IL-1β levels in the urine and acute polynephritis.
Mahyar, A. et al. Are serum procalcitonin and interleukin-1 beta suitable markers for diagnosis of acute pyelonephritis in children? Prague Med. Rep. 115, 16–23 (2014).
Kassir, K. et al. Cytokine profiles of pediatric patients treated with antibiotics for pyelonephritis: potential therapeutic impact. Clin. Diagn. Lab. Immunol. 8, 1060–1063 (2001).
Gürgöze, M. K. et al. Proinflammatory cytokines and procalcitonin in children with acute pyelonephritis. Pediatr. Nephrol. 20, 1445–1448 (2005).
Khalil, A. et al. Cytokine gene expression during experimental Escherichia coli pyelonephritis in mice. J. Urol. 158, 1576–1580 (1997). This paper highlights the production of IL-1β in an experimental model of acute pyelonephritis.
Hertting, O. et al. Enhanced chemokine response in experimental acute Escherichia coli pyelonephritis in IL-1 beta-deficient mice. Clin. Exp. Immunol. 131, 225–233 (2003).
Netea, M. G. et al. IL-1β processing in host defense: beyond the inflammasomes. PLoS Pathog. 6, e1000661 (2010).
Lukens, J. R. et al. Critical role for inflammasome-independent IL-1β production in osteomyelitis. Proc. Natl Acad. Sci. USA 111, 1066–1071 (2014).
Hughes, F. et al. The NLRP3 inflammasome mediates inflammation produced by bladder outlet obstruction. J. Urol. 195, 1598–1605 (2015).
Wright, K. J., Seed, P. C. & Hultgren, S. J. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 73, 7657–7668 (2005).
Rama, G., Chhina, D. K., Chhina, R. S. & Sharma, S. Urinary tract infections — microbial virulence determinants and reactive oxygen species. Comp. Immunol. Microbiol. Infect. Dis. 28, 339–349 (2005).
Hughes, F. M. Jr et al. The NACHT, NRR and PYD domains-containing protein 3 (NLRP3) inflammasome mediates inflammation and voiding dysfunction in a lipopolysaccharide-induced rat model of cystitis. J. Clin. Cell. Immunol. 7, 396 (2016). This paper explores a role for the NLRP3 inflammasome in LPS-mediated responses in the bladder.
Malley, S. E. & Vizzard, M. A. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol. Genomics 9, 5–13 (2002).
Acknowledgements
L.T. is now an employee of GlaxoSmithKline, UK. T.M. was supported by the Deutsche Forschungsgemeinschaft (DFG) grant MI471/6-1. Work in the laboratory of P.A. is supported by funds from The Wellcome Trust (108248/Z/15/Z), The Royal Society (RG150535) and core funds from Imperial College London.
Author information
Authors and Affiliations
Contributions
P.K.A., C.H., and L.T. researched data for the article. P.K.A. and C.H. wrote the manuscript. P.K.A. and T.M. took part in discussions of content. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
L.T. declares employment with GlaxoSmithKline, UK. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Hamilton, C., Tan, L., Miethke, T. et al. Immunity to uropathogens: the emerging roles of inflammasomes. Nat Rev Urol 14, 284–295 (2017). https://doi.org/10.1038/nrurol.2017.25
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2017.25
This article is cited by
-
Adapt(ed) to repair — TH2 immune responses in the bladder promote recurrent infections
Nature Immunology (2020)
-
Differential interleukin-1β induction by uropathogenic Escherichia coli correlates with its phylotype and serum C-reactive protein levels in Korean infants
Scientific Reports (2019)
-
A non-enzymatic method for dissection of mouse bladder urothelial tissue
Nature Protocols (2019)