Key Points
-
Testicular germ cell tumour (TGCT) has notably high heritability with commensurate high familial relative risks compared with other cancers
-
Genome-wide association studies have identified 25 loci for TGCT, consistent with a highly polygenic architecture underlying the genetic susceptibility
-
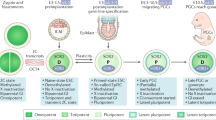
Intratubular germ cell neoplasia unclassified, the noninvasive precursor of TGCT, is molecularly similar to primordial germ cells and likely arises during fetal development, lying dormant in the gonads until puberty
-
Testicular tumours are mutationally stable and are characterized by KIT and KRAS mutations at modest frequency. Large-scale chromosomal gains are common in testicular tumours. Nearly all tumours carry isochromosome 12p, which is likely to be a key triggering event for malignant transformation
-
Platinum resistance affects <10% of patients but results in poor outcomes; although extensively investigated through somatic and germline pharmacogenomic studies, the mechanism remains obscure
Abstract
The genomic landscape of testicular germ cell tumour (TGCT) can be summarized using four overarching hypotheses. Firstly, TGCT risk is dominated by inherited genetic factors, which determine nearly half of all disease risk and are highly polygenic in nature. Secondly KIT–KITLG signalling is currently the major pathway that is implicated in TGCT formation, both as a predisposition risk factor and a somatic driver event. Results from genome-wide association studies have also consistently suggested that other closely related pathways involved in male germ cell development and sex determination are associated with TGCT risk. Thirdly, the method of disease formation is unique, with tumours universally stemming from a noninvasive precursor lesion, probably of fetal origin, which lies dormant through childhood into adolescence and then eventually begins malignant growth in early adulthood. Formation of a 12p isochromosome, a hallmark of TGCT observed in nearly all tumours, is likely to be a key triggering event for malignant transformation. Finally, TGCT have been shown to have a distinctive somatic mutational profile, with a low rate of point mutations contrasted with frequent large-scale chromosomal gains. These four hypotheses by no means constitute a complete model that explains TGCT tumorigenesis, but advances in genomic technologies have enabled considerable progress in describing and understanding the disease. Further advancing our understanding of the genomic basis of TGCT offers a clear opportunity for clinical benefit in terms of preventing invasive cancer arising in young men, decreasing the burden of chemotherapy-related survivorship issues and reducing mortality in the minority of patients who have treatment-refractory disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bray, F., Ferlay, J., Devesa, S. S., McGlynn, K. A. & Moller, H. Interpreting the international trends in testicular seminoma and nonseminoma incidence. Nat. Clin. Pract. Urol. 3, 532–543 (2006).
Ruf, C. G. et al. Changes in epidemiologic features of testicular germ cell cancer: age at diagnosis and relative frequency of seminoma are constantly and significantly increasing. Urol. Oncol. 32, 33.e1–33.e6 (2014).
Le Cornet, C. et al. Testicular cancer incidence to rise by 25% by 2025 in Europe? Model-based predictions in 40 countries using population-based registry data. Eur. J. Cancer 50, 831–839 (2014).
de Haas, E. C. et al. Early development of the metabolic syndrome after chemotherapy for testicular cancer. Ann. Oncol. 24, 749–755 (2013).
Bujan, L. et al. Impact of chemotherapy and radiotherapy for testicular germ cell tumors on spermatogenesis and sperm DNA: a multicenter prospective study from the CECOS network. Fertil. Steril. 100, 673–680 (2013).
Rusner, C. et al. Risk of second primary cancers after testicular cancer in East and West Germany: a focus on contralateral testicular cancers. Asian J. Androl. 16, 285–289 (2014).
Nitzsche, B. et al. Anti-tumour activity of two novel compounds in cisplatin-resistant testicular germ cell cancer. Br. J. Cancer 107, 1853–1863 (2012).
Swerdlow, A. J., De Stavola, B. L., Swanwick, M. A. & Maconochie, N. E. Risks of breast and testicular cancers in young adult twins in England and Wales: evidence on prenatal and genetic aetiology. Lancet 350, 1723–1728 (1997).
McGlynn, K. A., Devesa, S. S., Graubard, B. I. & Castle, P. E. Increasing incidence of testicular germ cell tumors among black men in the United States. J. Clin. Oncol. 23, 5757–5761 (2005).
Hemminki, K. & Li, X. Familial risk in testicular cancer as a clue to a heritable and environmental aetiology. Br. J. Cancer 90, 1765–1770 (2004).
Kharazmi, E. et al. Cancer risk in relatives of testicular cancer patients by histology type and age at diagnosis: a joint study from five Nordic countries. Eur. Urol. 68, 283–289 (2015).
Litchfield, K. et al. Quantifying the heritability of testicular germ cell tumour using both population-based and genomic approaches. Sci. Rep. 5, 13889 (2015).
Locatelli, I., Lichtenstein, P. & Yashin, A. I. The heritability of breast cancer: a Bayesian correlated frailty model applied to Swedish twins data. Twin Res. 7, 182–191 (2004).
Kampman, E. A first-degree relative with colorectal cancer: what are we missing? Cancer Epidemiol. Biomarkers Prev. 16, 1–3 (2007).
Crockford, G. P. et al. Genome-wide linkage screen for testicular germ cell tumour susceptibility loci. Hum. Mol. Genet. 15, 443–451 (2006).
Rapley, E. A. et al. Localization to Xq27 of a susceptibility gene for testicular germ-cell tumours. Nat. Genet. 24, 197–200 (2000).
Nathanson, K. L. et al. The Y deletion gr/gr and susceptibility to testicular germ cell tumor. Am. J. Hum. Genet. 77, 1034–1043 (2005).
Pathak, A. et al. Prospectively identified incident testicular cancer risk in a familial testicular cancer cohort. Cancer Epidemiol. Biomarkers Prev. 24, 1614–1621 (2015).
Rapley, E. A. et al. A genome-wide association study of testicular germ cell tumor. Nat. Genet. 41, 807–810 (2009).
Turnbull, C. & Rahman, N. Genome-wide association studies provide new insights into the genetic basis of testicular germ-cell tumour. Int. J. Androl. 34, e86–e96; discussion e96–e97 (2011).
Kanetsky, P. A. et al. Common variation in KITLG and at 5q31.3 predisposes to testicular germ cell cancer. Nat. Genet. 41, 811–815 (2009).
Turnbull, C. et al. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nat. Genet. 42, 604–607 (2010).
Kanetsky, P. A. et al. A second independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility. Hum. Mol. Genet. 20, 3109–3117 (2011).
Ruark, E. et al. Identification of nine new susceptibility loci for testicular cancer, including variants near DAZL and PRDM14. Nat. Genet. 45, 686–689 (2013).
Bojesen, S. E. et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat. Genet. 45, 371–384 (2013).
Chung, C. C. et al. Meta-analysis identifies four new loci associated with testicular germ cell tumor. Nat. Genet. 45, 680–685 (2013).
Litchfield, K. et al. Multi-stage genome wide association study identifies new susceptibility locus for testicular germ cell tumour on chromosome 3q25. Hum. Mol. Genet. 24, 1169–1176 (2015).
Kristiansen, W. et al. Two new loci and gene sets related to sex determination and cancer progression are associated with susceptibility to testicular germ cell tumor. Hum. Mol. Genet. 24, 4138–4146 (2015).
Chanock, S. High marks for GWAS. Nat. Genet. 41, 765–766 (2009).
Boldajipour, B. & Raz, E. What is left behind — quality control in germ cell migration. Sci. STKE 2007, e16 (2007).
Roskoski, R. Jr. Signaling by Kit protein-tyrosine kinase — the stem cell factor receptor. Biochem. Biophys. Res. Commun. 337, 1–13 (2005).
Heaney, J. D., Lam, M. Y., Michelson, M. V. & Nadeau, J. H. Loss of the transmembrane but not the soluble kit ligand isoform increases testicular germ cell tumor susceptibility in mice. Cancer Res. 68, 5193–5197 (2008).
Zeron-Medina, J. et al. A polymorphic p53 response element in KIT ligand influences cancer risk and has undergone natural selection. Cell 155, 410–422 (2013).
Sasaki, A. et al. Mammalian Sprouty4 suppresses Ras-independent ERK activation by binding to Raf1. Cell Cycle 2, 281–282 (2003).
Yan, W., Samson, M., Jegou, B. & Toppari, J. Bcl-w forms complexes with Bax and Bak, and elevated ratios of Bax/Bcl-w and Bak/Bcl-w correspond to spermatogonial and spermatocyte apoptosis in the testis. Mol. Endocrinol. 14, 682–699 (2000).
Yu, M. et al. The scaffolding adapter Gab2, via Shp-2, regulates kit-evoked mast cell proliferation by activating the Rac/JNK pathway. J. Biol. Chem. 281, 28615–28626 (2006).
Schrans-Stassen, B. H., Saunders, P. T., Cooke, H. J. & de Rooij, D. G. Nature of the spermatogenic arrest in Dazl-/- mice. Biol. Reprod. 65, 771–776 (2001).
Tsuneyoshi, N. et al. PRDM14 suppresses expression of differentiation marker genes in human embryonic stem cells. Biochem. Biophys. Res. Commun. 367, 899–905 (2008).
Smith, C. A., McClive, P. J., Western, P. S., Reed, K. J. & Sinclair, A. H. Conservation of a sex-determining gene. Nature 402, 601–602 (1999).
Krentz, A. D. et al. The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc. Natl Acad. Sci. USA 106, 22323–22328 (2009).
Litchfield, K., Shipley, J. & Turnbull, C. Common variants identified in genome-wide association studies of testicular germ cell tumour: an update, biological insights and clinical application. Andrology 3, 34–46 (2015).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
McGlynn, K. A. & Cook, M. B. Etiologic factors in testicular germ-cell tumors. Future Oncol. 5, 1389–1402 (2009).
Skakkebaek, N. E., Rajpert-De Meyts, E. & Main, K. M. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 16, 972–978 (2001).
Trabert, B., Zugna, D., Richiardi, L., McGlynn, K. A. & Akre, O. Congenital malformations and testicular germ cell tumors. Int. J. Cancer 133, 1900–1904 (2013).
Coffey, J. et al. Testicular microlithiasis as a familial risk factor for testicular germ cell tumour. Br. J. Cancer 97, 1701–1706 (2007).
Nizetic, D. & Groet, J. Tumorigenesis in Down's syndrome: big lessons from a small chromosome. Nat. Rev. Cancer 12, 721–732 (2012).
Hasle, H., Mellemgaard, A., Nielsen, J. & Hansen, J. Cancer incidence in men with Klinefelter syndrome. Br. J. Cancer 71, 416–420 (1995).
Litchfield, K. et al. Whole-exome sequencing reveals the mutational spectrum of testicular germ cell tumours. Nat. Commun. 6, 5973 (2015).
Stettner, A. R. et al. Familial ovarian germ cell cancer: report and review. Am. J. Med. Genet. 84, 43–46 (1999).
Kristensen, D. G., Skakkebaek, N. E., Rajpert-De Meyts, E. & Almstrup, K. Epigenetic features of testicular germ cell tumours in relation to epigenetic characteristics of foetal germ cells. Int. J. Dev. Biol. 57, 309–317 (2013).
Karlsson, R. et al. Investigation of six testicular germ cell tumor susceptibility genes suggests a parent-of-origin effect in SPRY4. Hum. Mol. Genet. 22, 3373–3380 (2013).
Oosterhuis, J. W. & Looijenga, L. H. Testicular germ-cell tumours in a broader perspective. Nat. Rev. Cancer 5, 210–222 (2005).
Kristensen, D. M. et al. Origin of pluripotent germ cell tumours: the role of microenvironment during embryonic development. Mol. Cell. Endocrinol. 288, 111–118 (2008).
Skakkebaek, N. E., Berthelsen, J. G., Giwercman, A. & Muller, J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int. J. Androl. 10, 19–28 (1987).
Rajpert-De Meyts, E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum. Reprod. Update 12, 303–323 (2006).
Jacobsen, G. K. & Henriques, U. V. A fetal testis with intratubular germ cell neoplasia (ITGCN). Mod. Pathol. 5, 547–549 (1992).
Biermann, K. et al. c-KIT is frequently mutated in bilateral germ cell tumours and down-regulated during progression from intratubular germ cell neoplasia to seminoma. J. Pathol. 213, 311–318 (2007).
Fossa, S. D. et al. Risk of contralateral testicular cancer: a population-based study of 29,515 U.S. men. J. Natl Cancer Inst. 97, 1056–1066 (2005).
Coffey, J. et al. Somatic KIT mutations occur predominantly in seminoma germ cell tumors and are not predictive of bilateral disease: report of 220 tumors and review of literature. Genes Chromosomes Cancer 47, 34–42 (2008).
Horwich, A., Shipley, J. & Huddart, R. Testicular germ-cell cancer. Lancet 367, 754–765 (2006).
Rajpert-De Meyts, E. et al. The emerging phenotype of the testicular carcinoma in situ germ cell. APMIS 111, 267–278; discussion 278–279 (2003).
Dieckmann, K. P., Kulejewski, M., Heinemann, V. & Loy, V. Testicular biopsy for early cancer detection — objectives, technique and controversies. Int. J. Androl. 34, e7–e13 (2011).
Almstrup, K. et al. Genome-wide gene expression profiling of testicular carcinoma in situ progression into overt tumours. Br. J. Cancer 92, 1934–1941 (2005).
Biermann, K. et al. Genome-wide expression profiling reveals new insights into pathogenesis and progression of testicular germ cell tumors. Cancer Genomics Proteomics 4, 359–367 (2007).
Gori, S. et al. Germ cell tumours of the testis. Crit. Rev. Oncol. Hematol. 53, 141–164 (2005).
Mai, P. L. et al. The International Testicular Cancer Linkage Consortium: a clinicopathologic descriptive analysis of 461 familial malignant testicular germ cell tumor kindred. Urol. Oncol. 28, 492–499 (2010).
Forman, D. et al. Familial testicular cancer: a report of the UK family register, estimation of risk and an HLA class 1 sib-pair analysis. Br. J. Cancer 65, 255–262 (1992).
Cutcutache, I. et al. Exome-wide sequencing shows low mutation rates and identifies novel mutated genes in seminomas. Eur. Urol. 68, 77–83 (2015).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
McIntyre, A. et al. Amplification and overexpression of the KIT gene is associated with progression in the seminoma subtype of testicular germ cell tumors of adolescents and adults. Cancer Res. 65, 8085–8089 (2005).
Kemmer, K. et al. KIT mutations are common in testicular seminomas. Am. J. Pathol. 164, 305–313 (2004).
Kim, H. J. et al. KIT D816 mutation associates with adverse outcomes in core binding factor acute myeloid leukemia, especially in the subgroup with RUNX1/RUNX1T1 rearrangement. Ann. Hematol. 92, 163–171 (2013).
Bignell, G. et al. Sequence analysis of the protein kinase gene family in human testicular germ-cell tumors of adolescents and adults. Genes Chromosomes Cancer 45, 42–46 (2006).
Heidenreich, A. et al. Immunohistochemical and mutational analysis of the p53 tumour suppressor gene aml the bcl-2 oncogene in primary testicular germ cell, tumours. APMIS 106, 90–99 (1998).
Laumann, R., Jucker, M. & Tesch, H. Point mutations in the conserved regions of the p53 tumor suppressor gene do not account for the transforming process in the Jurkat acute lymphoblastic leukemia T-cells. Leukemia 6, 227–228 (1992).
Voorhoeve, P. M. et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell 124, 1169–1181 (2006).
Li, B., Cheng, Q., Li, Z. & Chen, J. p53 inactivation by MDM2 and MDMX negative feedback loops in testicular germ cell tumors. Cell Cycle 9, 1411–1420 (2010).
Suijkerbuijk, R. F. et al. Overrepresentation of chromosome 12p sequences and karyotypic evolution in i(12p)-negative testicular germ-cell tumors revealed by fluorescence in situ hybridization. Cancer Genet. Cytogenet. 70, 85–93 (1993).
Atkin, N. B. & Baker, M. C. Specific chromosome change, i(12p), in testicular-tumors. Lancet 2, 1349–1349 (1982).
Atkin, N. B. & Baker, M. C. i(12p): specific chromosomal marker in seminoma and malignant teratoma of the testis. Cancer Genet. Cytogenet. 10, 199–204 (1983).
Sandberg, A. A., Meloni, A. M. & Suijkerbuijk, R. F. Reviews of chromosome studies in urological tumors.3. Cytogenetics and genes in testicular tumors. J. Urol. 155, 1531–1556 (1996).
Rodriguez, S. et al. Expression profile of genes from 12p in testicular germ cell tumors of adolescents and adults associated with i(12p) and amplification at 12p11.2–p12.1. Oncogene 22, 1880–1891 (2003).
Korkola, J. E. et al. Down-regulation of stem cell genes, including those in a 200-kb gene cluster at 12p13.31, is associated with in vivo differentiation of human male germ cell tumors. Cancer Res. 66, 820–827 (2006).
Ottesen, A. M. et al. High-resolution comparative genomic hybridization detects extra chromosome arm 12p material in most cases of carcinoma in situ adjacent to overt germ cell tumors, but not before the invasive tumor development. Genes Chromosomes Cancer 38, 117–125 (2003).
Summersgill, B., Osin, P. S., Lu, Y. J., Huddart, R. & Shipley, J. Chromosomal imbalances associated with carcinoma in situ and associated testicular germ cell tumours of adolescents and adults. Br. J. Cancer 85, 213–219 (2001).
Henegariu, O., Vance, G. H., Heiber, D., Pera, M. & Heerema, N. A. Triple-color FISH analysis of 12p amplification in testicular germ-cell tumors using 12p band-specific painting probes. J. Mol. Med. (Berl.) 76, 648–655 (1998).
Roelofs, H. et al. Restricted 12p amplification and RAS mutation in human germ cell tumors of the adult testis. Am. J. Pathol. 157, 1155–1166 (2000).
Summersgill, B. et al. Molecular cytogenetic analysis of adult testicular germ cell tumours and identification of regions of consensus copy number change. Br. J. Cancer 77, 305–313 (1998).
Zafarana, G. et al. 12p-amplicon structure analysis in testicular germ cell tumors of adolescents and adults by array CGH. Oncogene 22, 7695–7701 (2003).
LeBron, C. et al. Genome-wide analysis of genetic alterations in testicular primary seminoma using high resolution single nucleotide polymorphism arrays. Genomics 97, 341–349 (2011).
Brown, P. R., Miki, K., Harper, D. B. & Eddy, E. M. A-kinase anchoring protein 4 binding proteins in the fibrous sheath of the sperm flagellum. Biol. Reprod. 68, 2241–2248 (2003).
Chemes, H. E., Brugo, S., Zanchetti, F., Carrere, C. & Lavieri, J. C. Dysplasia of the fibrous sheath: an ultrastructural defect of human spermatozoa associated with sperm immotility and primary sterility. Fertil. Steril. 48, 664–669 (1987).
Miki, K. et al. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev. Biol. 248, 331–342 (2002).
Vladusic, T. et al. Histological groups of human postpubertal testicular germ cell tumours harbour different genetic alterations. Anticancer Res. 34, 4005–4012 (2014).
Oldenburg, J. et al. Testicular seminoma and non-seminoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24, 125–132 (2013).
Siegel, R. et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clinicians 62, 220–241 (2012).
Usanova, S. et al. Cisplatin sensitivity of testis tumour cells is due to deficiency in interstrand-crosslink repair and low ERCC1-XPF expression. Mol.Cancer 9, 248 (2010).
Cavallo, F. et al. Reduced proficiency in homologous recombination underlies the high sensitivity of embryonal carcinoma testicular germ cell tumors to cisplatin and poly (ADP-ribose) polymerase inhibition. PLoS ONE 7, e51563 (2012).
Gutekunst, M. et al. p53 hypersensitivity is the predominant mechanism of the unique responsiveness of testicular germ cell tumor (TGCT) cells to cisplatin. PLoS ONE 6, e19198 (2011).
Sheikine, Y. et al. Molecular genetics of testicular germ cell tumors. Am. J. Cancer Res. 2, 153–167 (2012).
Honecker, F. et al. Microsatellite instability, mismatch repair deficiency, and BRAF mutation in treatment-resistant germ cell tumors. J. Clin. Oncol. 27, 2129–2136 (2009).
Feldman, D. R. et al. Presence of somatic mutations within PIK3CA, AKT, RAS, and FGFR3 but not BRAF in cisplatin-resistant germ cell tumors. Clin. Cancer Res. 20, 3712–3720 (2014).
Van Allen, E. M. et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 4, 1140–1153 (2014).
Einhorn, L. H., Brames, M. J., Heinrich, M. C., Corless, C. L. & Madani, A. Phase II study of imatinib mesylate in chemotherapy refractory germ cell tumors expressing KIT. Am. J. Clin. Oncol. 29, 12–13 (2006).
Noel, E. E. et al. The association of CCND1 overexpression and cisplatin resistance in testicular germ cell tumors and other cancers. Am. J. Pathol. 176, 2607–2615 (2010).
Garcia-Velasco, A. et al. Biological markers of cisplatin resistance in advanced testicular germ cell tumours. Clin. Transl. Oncol. 14, 452–457 (2012).
de Haas, E. C. et al. Association of PAI-1 gene polymorphism with survival and chemotherapy-related vascular toxicity in testicular cancer. Cancer 116, 5628–5636 (2010).
Singhera, M., Lees, K., Huddart, R. & Horwich, A. Minimizing toxicity in early-stage testicular cancer treatment. Expert Rev. Anticancer Ther. 12, 185–193 (2012).
Oldenburg, J. et al. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and-M1, a retrospective cross sectional study. J. Transl. Med. 5, 70 (2007).
Peters, U. et al. Glutathione S-transferase genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Drugs 11, 639–643 (2000).
Huddart, R. A. et al. Fertility, gonadal and sexual function in survivors of testicular cancer. Br. J. Cancer 93, 200–207 (2005).
O'Flaherty, C., Hales, B. F., Chan, P. & Robaire, B. Impact of chemotherapeutics and advanced testicular cancer or Hodgkin lymphoma on sperm deoxyribonucleic acid integrity. Fertil. Steril. 94, 1374–1379 (2010).
Travis, L. B. et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J. Natl Cancer Inst. 97, 1354–1365 (2005).
Greene, M. H. et al. Familial testicular germ cell tumors (FTGCT) — overview of a multidisciplinary etiologic study. Andrology 3, 47–58 (2015).
Bahcall, O. Risk prediction and population screening for breast, ovarian and prostate cancers. Nat. Genet. http://dx.doi.org/10.1038/ngicogs.5 (2012).
Litchfield, K. et al. Polygenic susceptibility to testicular cancer: implications for personalised health care. Br. J. Cancer 113, 1512–1518 (2015).
Almstrup, K. et al. Screening of subfertile men for testicular carcinoma in situ by an automated image analysis-based cytological test of the ejaculate. Int. J. Androl 34, e21–e30; discussion e30–e30 (2011).
Hoei-Hansen, C. E. et al. Towards a non-invasive method for early detection of testicular neoplasia in semen samples by identification of fetal germ cell-specific markers. Hum. Reprod. 22, 167–173 (2007).
Skol, A. D., Scott, L. J., Abecasis, G. R. & Boehnke, M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat. Genet. 38, 390–390 (2006).
Nelson, M. R. et al. The support of human genetic evidence for approved drug indications. Nat. Genet. 47, 856–860 (2015).
Litchfield, K. Abstract 2986: meta-analysis of whole exome sequencing data reveals the mutational spectrum of testicular germ cell tumors. Cancer Res. 75, 2986 (2015).
Basten, S. G. et al. Mutations in LRRC50 predispose zebrafish and humans to seminomas. PLoS Genet. 9, e1003384 (2013).
Fukuda, S. et al. Expression of vascular endothelial growth factor in patients with testicular germ cell tumors as an indicator of metastatic disease. Cancer 85, 1323–1330 (1999).
Nieto, Y. et al. Bevacizumab/high-dose chemotherapy with autologous stem-cell transplant for poor-risk relapsed or refractory germ-cell tumors. Ann. Oncol. http://dx.doi.org/10.1093/annonc/mdv310 (2015).
Fankhauser, C. D. et al. Frequent PD-L1 expression in testicular germ cell tumors. Br. J. Cancer 113, 411–413 (2015).
Szklarczyk, D. et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452 (2015).
Litchfield, K. et al. Identification of four new susceptibility loci for testicular germ cell tumour. Nat. Commun. 6, 8690 (2015).
Author information
Authors and Affiliations
Contributions
K.L. and M.L. researched data for the article, R.A.H., J.S. and C.T. contributed to discussion of its contents. K.L. wrote the manuscript and K.L., M.L. and C.T. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Litchfield, K., Levy, M., Huddart, R. et al. The genomic landscape of testicular germ cell tumours: from susceptibility to treatment. Nat Rev Urol 13, 409–419 (2016). https://doi.org/10.1038/nrurol.2016.107
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2016.107
This article is cited by
-
Germline stem cells in human
Signal Transduction and Targeted Therapy (2022)
-
DNA replication machinery prevents Rad52-dependent single-strand annealing that leads to gross chromosomal rearrangements at centromeres
Communications Biology (2020)
-
MicroRNA and transcription factor co-regulatory networks and subtype classification of seminoma and non-seminoma in testicular germ cell tumors
Scientific Reports (2020)
-
gr/gr deletion predisposes to testicular germ cell tumour independently from altered spermatogenesis: results from the largest European study
European Journal of Human Genetics (2019)