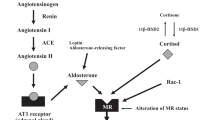
Abstract
Refractory nephrotic syndrome continues to be a therapeutic challenge despite advances in immunosuppression and blockade of the renin–angiotensin–aldosterone cascade. Adrenocorticotropic hormone (ACTH), a pituitary neuroimmunoendocrine polypeptide, was widely used in the 1950s as an effective therapy for childhood nephrotic syndrome, but has since been replaced by synthetic glucocorticoid analogues. In addition to controlling steroidogenesis, ACTH also acts as an important physiological agonist of the melanocortin system. Clinical and experimental evidence now suggests that ACTH has antiproteinuric, lipid-lowering and renoprotective properties, which are not fully explained by its steroidogenic effects. ACTH therapy is effective in inducing remission of nephrotic syndrome in patients with a variety of proteinuric nephropathies, even those resistant to steroids and other immunosuppressants. This Perspectives article describes the biophysiology of ACTH, with an emphasis on its melanocortin actions, particularly in renal parenchymal cells, which could potentially explain the therapeutic effects of ACTH in nephrotic glomerulopathies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dores, R. M. Adrenocorticotropic hormone, melanocyte-stimulating hormone, and the melanocortin receptors: revisiting the work of Robert Schwyzer: a thirty-year retrospective. Ann. NY Acad. Sci. 1163, 93–100 (2009).
Arneil, G. C. & Wilson, H. E. A.C.T.H. in nephrosis. Arch. Dis. Child. 28, 372–380 (1953).
Piel, C. F. Management of nephrosis; the use of long continued hormone therapy. Calif. Med. 85, 152–156 (1956).
Lauson, H. D., Forman, C. W., Mc, N. H., Mattar, G. & Barnett, H. L. The effect of corticotropin (ACTH) on glomerular permeability to albumin in children with the nephrotic syndrome. J. Clin. Invest. 33, 657–664 (1954).
Berg, A. L. & Arnadottir, M. ACTH revisited--potential implications for patients with renal disease. Nephrol. Dial. Transplant. 15, 940–942 (2000).
Ponticelli, C. Membranous nephropathy. J. Nephrol. 20, 268–287 (2007).
Cone, R. D. Studies on the physiological functions of the melanocortin system. Endocr. Rev. 27, 736–749 (2006).
Voisey, J., Carroll, L. & van Daal, A. Melanocortins and their receptors and antagonists. Curr. Drug Targets 4, 586–597 (2003).
Penhoat, A., Naville, D. & Begeot, M. The adrenocorticotropic hormone receptor. Curr. Opin. Endocrinol. Diabetes 8, 112–117 (2001).
Lindskog, A. et al. Melanocortin 1 receptor agonists reduce proteinuria. J. Am. Soc. Nephrol. 21, 1290–1298 (2010).
Gong, R. & Dworkin, L. D. ACTH (Acthar Gel) prevents proteinuria and renal injury in the remnant kidney: Evidence for direct podocyte protection. J. Am. Soc. Nephrol. 21, 548A (2010).
Chhajlani, V. Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem. Mol. Biol. Int. 38, 73–80 (1996).
Sanchez, E., Rubio, V. C. & Cerda-Reverter, J. M. Characterization of the sea bass melanocortin 5 receptor: a putative role in hepatic lipid metabolism. J. Exp. Biol. 212, 3901–3910 (2009).
Ni, X. P., Bhargava, A., Pearce, D. & Humphreys, M. H. Modulation by dietary sodium intake of melanocortin 3 receptor mRNA and protein abundance in the rat kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R560–R567 (2006).
Lee, Y. S., Park, J. J. & Chung, K. Y. Change of melanocortin receptor expression in rat kidney ischemia-reperfusion injury. Transplant. Proc. 40, 2142–2144 (2008).
Schwandt, P. & Richter, W. O. Effects of pituitary peptides on fat mobilisation. Int. J. Obes. 6 (Suppl. 1), 49–54 (1982).
Berg, A. L., Hansson, P. & Nilsson-Ehle, P. ACTH 1–24 decreases hepatic lipase activities and low density lipoprotein concentrations in healthy men. J. Intern. Med. 229, 201–203 (1991).
Berg, A. L. & Nilsson-Ehle, P. ACTH lowers serum lipids in steroid-treated hyperlipemic patients with kidney disease. Kidney Int. 50, 538–542 (1996).
Beloff-Chain, A., Morton, J., Dunmore, S., Taylor, G. W. & Morris, H. R. Evidence that the insulin secretagogue, beta-cell-tropin, is ACTH22–39. Nature 301, 255–258 (1983).
Schoneshofer, M. & Goverde, H. J. Corticotropin in human plasma. General considerations. Surv. Immunol. Res. 3, 55–63 (1984).
Wan, L., Chen, Y. H. & Chang, T. W. Improving pharmacokinetic properties of adrenocorticotropin by site-specific lipid modification. J. Pharm. Sci. 92, 1882–1892 (2003).
Kohda, Y., Chiao, H. & Star, R. A. alpha-Melanocyte-stimulating hormone and acute renal failure. Curr. Opin. Nephrol. Hypertens. 7, 413–417 (1998).
Chiao, H. et al. Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats. J. Clin. Invest. 99, 1165–1172 (1997).
Kolgazi, M., Arbak, S. & Alican, I. The effect of alpha-melanocyte stimulating hormone on gentamicin-induced acute nephrotoxicity in rats. J. Appl. Toxicol. 27, 183–188 (2007).
Li, C. et al. alpha-MSH prevents impairment in renal function and dysregulation of AQPs and Na-K-ATPase in rats with bilateral ureteral obstruction. Am. J. Physiol. Renal. Physiol. 290, F384–F396 (2006).
Gong, R., Ge, Y., Tolbert, E. M., Zhuang, S. & Dworkin, L. D. Renoprotection by adrenocorticotropin in experimental acute kidney injury. J. Am. Soc. Nephrol. 20, 510A (2009).
Garcia, D. L., Rennke, H. G., Brenner, B. M. & Anderson, S. Chronic glucocorticoid therapy amplifies glomerular injury in rats with renal ablation. J. Clin. Invest. 80, 867–874 (1987).
Berg, A. L. & Arnadottir, M. ACTH-induced improvement in the nephrotic syndrome in patients with a variety of diagnoses. Nephrol. Dial. Transplant. 19, 1305–1307 (2004).
Berg, A. L., Nilsson-Ehle, P. & Arnadottir, M. Beneficial effects of ACTH on the serum lipoprotein profile and glomerular function in patients with membranous nephropathy. Kidney Int. 56, 1534–1543 (1999).
Ponticelli, C. et al. A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am. J. Kidney Dis. 47, 233–240 (2006).
Rauen, T., Michaelis, A., Floege, J. & Mertens, P. R. Case series of idiopathic membranous nephropathy with long-term beneficial effects of ACTH peptide 1–24. Clin. Nephrol. 71, 637–642 (2009).
Picardi, L. et al. ACTH therapy in nephrotic syndrome induced by idiopathic membranous nephropathy. Clin. Nephrol. 62, 403–404 (2004).
Bomback, A. S. et al. Treatment of resistant nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Des. Develop. Ther. 5, 147–153 (2011).
Beck, L. H. et al. Treatment of idiopathic membranous nephropathy with ACTH is associated with a decline in anti-PLA2R antibody. J. Am. Soc. Nephrol. 20, 306A (2009).
Beck, L. H. Jr et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361, 11–21 (2009).
Rosenblum, A. H. & Rosenblum, P. Anaphylactic reactions to adrenocorticotropic hormone in children. J. Pediatr. 64, 387–395 (1964).
Glassock, R. J. The treatment of idiopathic membranous nephropathy: a dilemma or a conundrum? Am. J. Kidney Dis. 44, 562–566 (2004).
Catania, A. The melanocortin system in leukocyte biology. J. Leukoc. Biol. 81, 383–392 (2007).
Symmers, W. S. Thrombotic microangiopathic haemolytic anaemia (thrombotic microangiopathy). Br. Med. J. 2, 897–903 (1952).
Catania, A., Gatti, S., Colombo, G. & Lipton, J. M. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol. Rev. 56, 1–29 (2004).
Remuzzi, G. & Bertani, T. Pathophysiology of progressive nephropathies. N. Engl. J. Med. 339, 1448–1456 (1998).
Johnson, E. W., Blalock, J. E. & Smith, E. M. ACTH receptor-mediated induction of leukocyte cyclic AMP. Biochem. Biophys. Res. Comm. 157, 1205–1211 (1988).
Lepique, A. P. et al. c-Myc protein is stabilized by fibroblast growth factor 2 and destabilized by ACTH to control cell cycle in mouse Y1 adrenocortical cells. J. Mol. Endocrinol. 33, 623–638 (2004).
Wang, H., Jia, Z., Liu, G. & Yang, T. Adrenocorticotropic hormone attenuates diabetic nephropathy in Zucker diabetic fatty rats via inhibition of oxidative and inflammatory response. J. Am. Soc. Nephrol. 21, 825A (2010).
Roth, J. A., Kaeberle, M. L. & Hsu, W. H. Effects of ACTH administration on bovine polymorphonuclear leukocyte function and lymphocyte blastogenesis. Am. J. Vet. Res. 43, 412–416 (1982).
Smith, E., Hammarstrom, L., Moller, E. & Matell, G. The effect of ACTH treatment on lymphocyte subpopulations in patients with myasthenia gravis. Neurology 26, 915–918 (1976).
Cooper, A. et al. Alpha-melanocyte-stimulating hormone suppresses antigen-induced lymphocyte proliferation in humans independently of melanocortin 1 receptor gene status. J. Immunol. 175, 4806–4813 (2005).
Andersen, G. N. et al. Quantitative measurement of the levels of melanocortin receptor subtype 1, 2, 3 and 5 and pro-opio-melanocortin peptide gene expression in subsets of human peripheral blood leucocytes. Scand. J. Immunol. 61, 279–284 (2005).
Gonzalez-Rey, E., Chorny, A. & Delgado, M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat. Rev. Immunol. 7, 52–63 (2007).
Koyama, A., Fujisaki, M., Kobayashi, M., Igarashi, M. & Narita, M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 40, 453–460 (1991).
Tracey, K. J. The inflammatory reflex. Nature 420, 853–859 (2002).
Yeboah, M. M. et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 74, 62–69 (2008).
Guarini, S. et al. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc. Res. 63, 357–365 (2004).
Cases, A. & Coll, E. Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int. Suppl. 68, S87–S93 (2005).
Rayner, B. L., Byrne, M. J. & van Zyl Smit, R. A prospective clinical trial comparing the treatment of idiopathic membranous nephropathy and nephrotic syndrome with simvastatin and diet, versus diet alone. Clin. Nephrol. 46, 219–224 (1996).
Rabelink, A. J., Hene, R. J., Erkelens, D. W., Joles, J. A. & Koomans, H. A. Partial remission of nephrotic syndrome in patient on long-term simvastatin. Lancet 335, 1045–1046 (1990).
Thomas, M. E. et al. Simvastatin therapy for hypercholesterolemic patients with nephrotic syndrome or significant proteinuria. Kidney Int. 44, 1124–1129 (1993).
Chan, P. C. et al. Lovastatin in glomerulonephritis patients with hyperlipidaemia and heavy proteinuria. Nephrol. Dial. Transplant. 7, 93–99 (1992).
Ghiggeri, G. M. et al. Depletion of clusterin in renal diseases causing nephrotic syndrome. Kidney Int. 62, 2184–2194 (2002).
Candiano, G. et al. Apolipoproteins prevent glomerular albumin permeability induced in vitro by serum from patients with focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 12, 143–150 (2001).
Berg, A. L. & Nilsson-Ehle, P. Direct effects of corticotropin on plasma lipoprotein metabolism in man--studies in vivo and in vitro. Metabolism 43, 90–97 (1994).
Rastaldi, M. P. et al. Glomerular clusterin is associated with PKC-alpha/beta regulation and good outcome of membranous glomerulonephritis in humans. Kidney Int. 70, 477–485 (2006).
Acknowledgements
Research work by R. Gong has been supported by research grants from Questcor, Foundation for Health, and NIH Grant R01DK092485.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
R. Gong declares that he has received research grants from Questcor.
Rights and permissions
About this article
Cite this article
Gong, R. The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol 8, 122–128 (2012). https://doi.org/10.1038/nrneph.2011.190
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2011.190
This article is cited by
-
How immunosuppressive drugs may directly target podocytes in glomerular diseases
Pediatric Nephrology (2022)
-
Repository corticotropin injection versus corticosteroids for protection against renal damage in a focal segmental glomerulosclerosis rodent model
BMC Nephrology (2020)
-
Melanocortin 5 receptor signaling pathway in health and disease
Cellular and Molecular Life Sciences (2020)
-
Use of synthetic adrenocorticotropic hormone in patients with IgA nephropathy
BMC Nephrology (2018)
-
Treatment of primary membranous nephropathy: where are we now?
Journal of Nephrology (2018)