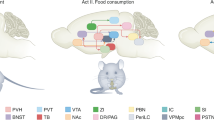
Key Points
-
Common brain sites and neurotransmitter systems are activated in response to palatable food and drugs of abuse. Addictive drugs seem to be particularly effective in stimulating the very same brain systems whose function is to process information related to food palatability and regulate the incentive value of food.
-
The nucleus tractus solitarius (NTS) receives input related to food palatability from the mouth and gastrointestinal tract. Circulating hormonal regulators of hunger and satiety also influence NTS activity. The NTS contains at least three distinct populations of neurons, those containing catecholamines, proopiomelanocortin (POMC) or glucagon-like peptide 1 (GLP1; a cleavage product of glucagon). The NTS seems to play a key part in drug reward and also in some aspects of drug withdrawal.
-
The insular cortex encodes and stores information related to the valence and magnitude of food and drug reward. In particular, the insula seems to play an important part in contrasting the palatability of currently and previously available food or drug reinforcers and thereby determining if there has been a change in the relative value of food or drug reinforcers.
-
The orbitofrontal cortex (OFC) processes information about the relative motivational value of palatable food or addictive drugs at any given time based on information from metabolic or hedonic circuitries in the brain. As such, the OFC seems to have a key role in the development of satiety.
-
The striatum, and in particular dopamine, opioid and cannabinoid systems within the striatum, encode information related to the appetitive and incentive value of food and drugs of abuse.
-
Palatable food and drugs of abuse can trigger common molecular adaptations in brain reward systems, including increases in the transcription factor ΔFOSB. Such neuroadaptive responses are likely to contribute to the development of obesity and addiction.
-
Brain inflammatory responses have been implicated in the development of obesity and drug addiction. The transcription factor nuclear factor-κB (NF-κB) in particular may have a key role in driving excessive food or drug intake.
-
New areas of research in obesity and drug addiction include assessing the role for neurogenesis in the adult brain and the involvement of nuclear hormone receptors like peroxisome proliferator-activated receptor-γ (PPARγ). In addition, gene regulatory processes including DNA methylation and chromatin modifications, and post-transcriptional gene regulatory processes like RNA editing and microRNAs are also emerging as important regulators of vulnerability to obesity and drug addiction.
Abstract
The hedonic properties of food can stimulate feeding behaviour even when energy requirements have been met, contributing to weight gain and obesity. Similarly, the hedonic effects of drugs of abuse can motivate their excessive intake, culminating in addiction. Common brain substrates regulate the hedonic properties of palatable food and addictive drugs, and recent reports suggest that excessive consumption of food or drugs of abuse induces similar neuroadaptive responses in brain reward circuitries. Here, we review evidence suggesting that obesity and drug addiction may share common molecular, cellular and systems-level mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kenny, P. J. Reward mechanisms in obesity: new insights and future directions. Neuron 69, 664–679 (2011).
Wyrwicka, W., Dobrzecka, C. & Tarnecki, R. On the instrumental conditioned reaction evoked by electrical stimulation of the hypothalamus. Science 130, 336–337 (1959).
Will, M. J., Pratt, W. E. & Kelley, A. E. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiol. Behav. 89, 226–234 (2006).
McCrory, M. A., Suen, V. M. & Roberts, S. B. Biobehavioral influences on energy intake and adult weight gain. J. Nutr. 132, 3830S–3834S (2002).
Kelly, M. T. et al. Increased portion size leads to a sustained increase in energy intake over 4 d in normal-weight and overweight men and women. Br. J. Nutr. 102, 470–477 (2009).
Benton, D. The plausibility of sugar addiction and its role in obesity and eating disorders. Clin. Nutr. 29, 288–303 (2010).
Corsica, J. A. & Pelchat, M. L. Food addiction: true or false? Curr. Opin. Gastroenterol. 26, 165–169 (2010).
Warwick, Z. S. Probing the causes of high-fat diet hyperphagia: a mechanistic and behavioral dissection. Neurosci. Biobehav. Rev. 20, 155–161 (1996).
Schwartz, G. J. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition 16, 866–873 (2000).
Rolls, E. T. Brain mechanisms underlying flavour and appetite. Phil. Trans. R Soc. Lond. Series B 361, 1123–1136 (2006). An excellent overview of the neurocircuitries that regulate the perception of food palatability.
Small, D. M., Zatorre, R. J., Dagher, A., Evans, A. C. & Jones-Gotman, M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain 124, 1720–1733 (2001). An important paper that identifies brain systems that are involved in the development of satiety and sites that are recruited to limit further consumption.
Volkow, N. D., Wang, G. J. & Baler, R. D. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn. Sci. 15, 37–46 (2011).
Appleyard, S. M. et al. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J. Neurosci. 27, 13292–13302 (2007).
Covasa, M. & Ritter, R. C. Reduced sensitivity to the satiation effect of intestinal oleate in rats adapted to high-fat diet. Am. J. Physiol. 277, R279–R285 (1999).
Donovan, M. J., Paulino, G. & Raybould, H. E. Activation of hindbrain neurons in response to gastrointestinal lipid is attenuated by high fat, high energy diets in mice prone to diet-induced obesity. Brain Res. 1248, 136–140 (2009).
Smith, R. J. & Aston-Jones, G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct. Funct. 213, 43–61 (2008).
Koob, G. & Kreek, M. J. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry 164, 1149–1159 (2007).
Simons, C. T., Boucher, Y., Carstens, M. I. & Carstens, E. Nicotine suppression of gustatory responses of neurons in the nucleus of the solitary tract. J. Neurophysiol. 96, 1877–1886 (2006).
Wise, R. A. & Kiyatkin, E. A. Differentiating the rapid actions of cocaine. Nature Rev. Neurosci. 12, 479–484 (2011).
Lenoir, M. & Kiyatkin, E. A. Critical role of peripheral actions of intravenous nicotine in mediating its central effects. Neuropsychopharmacology 36, 2125–2138 (2011). An important paper demonstrating that non-brain actions of nicotine may contribute to its reinforcing properties. It suggests that addictive drugs may act through peripheral mechanisms to trigger addiction.
Olson, V.G. et al. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science 311, 1017–1020 (2006).
Delfs, J. M., Zhu, Y., Druhan, J. P. & Aston-Jones, G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature 403, 430–434 (2000).
Harris, G. C. & Aston-Jones, G. Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behav. Brain Res. 176, 251–258 (2007).
Garcia-Diaz, D. E., Jimenez-Montufar, L. L., Guevara-Aguilar, R., Wayner, M. J. & Armstrong, D. L. Olfactory and visceral projections to the nucleus of the solitary tract. Physiol. Behav. 44, 619–624 (1988).
Ziomber, A. et al. Magnetically induced vagus nerve stimulation and feeding behavior in rats. J. Physiol. Pharmacol. 60, 71–77 (2009).
Burneo, J. G., Faught, E., Knowlton, R., Morawetz, R. & Kuzniecky, R. Weight loss associated with vagus nerve stimulation. Neurology 59, 463–464 (2002).
Wang, G. J. et al. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc. Natl Acad. Sci. USA 103, 15641–15645 (2006).
Ertelt, T. W. et al. Alcohol abuse and dependence before and after bariatric surgery: a review of the literature and report of a new data set. Surg. Obes. Relat. Dis. 4, 647–650 (2008).
Cunningham, J. T., Mifflin, S. W., Gould, G. G. & Frazer, A. Induction of cFos and ΔFosB immunoreactivity in rat brain by Vagal nerve stimulation. Neuropsychopharmacology 33, 1884–1895 (2008).
Nunez, C. et al. Induction of FosB/ΔFosB in the brain stress system-related structures during morphine dependence and withdrawal. J. Neurochem. 114, 475–487 (2010).
Mumberg, D., Lucibello, F. C., Schuermann, M. & Muller, R. Alternative splicing of fosB transcripts results in differentially expressed mRNAs encoding functionally antagonistic proteins. Genes Dev. 5, 1212–1223 (1991).
McClung, C. A. & Nestler, E. J. Regulation of gene expression and cocaine reward by CREB and ΔFosB. Nature Neurosci. 6, 1208–1215 (2003).
Appleyard, S. M. et al. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J. Neurosci. 25, 3578–3585 (2005).
Zhang, Y. et al. Pro-opiomelanocortin gene transfer to the nucleus of the solitary track but not arcuate nucleus ameliorates chronic diet-induced obesity. Neuroscience 169, 1662–1671 (2010).
Holst, J. J. The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 (2007).
Turton, M. D. et al. A role for glucagon-like peptide1 in the central regulation of feeding. Nature 379, 69–72 (1996). An important paper showing that GLP1 that is produced in the NTS can control food intake. Further studies will be necessary to determine whether GLP1 also regulates drug intake.
Hayes, M. R., Bradley, L. & Grill, H. J. Endogenous hindbrain glucagon-like peptide1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150, 2654–2659 (2009).
Barrera, J. G. et al. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide1 loss of function. J. Neurosci. 31, 3904–3913 (2011).
Hayes, M. R. et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide1 receptor activation. Cell Metab. 13, 320–330 (2011).
Paulus, M. P. Neural basis of reward and craving-a homeostatic point of view. Dialogues Clin. Neurosci. 9, 379–387 (2007).
Johnson, P. M. & Kenny, P. J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neurosci. 13, 635–641 (2010). This paper shows that consumption of palatable food can become compulsive in much the same way that consumption of addictive drugs can be compulsive. It supports the hypothesis that obesity and addiction share common underlying mechanisms.
Cottone, P., Sabino, V., Steardo, L. & Zorrilla, E. P. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology 33, 524–535 (2008). This paper shows that rats will shift their consummatory preference to the most palatable item available and will reject a less palatable alternative, even one that they previously readily consumed, after a period of exposure to the more palatable item. The authors show that this so-called negative contrast effect is regulated by opioid receptors.
Lin, J. Y., Roman, C. & Reilly, S. Insular cortex and consummatory successive negative contrast in the rat. Behav. Neurosci. 123, 810–814 (2009).
Reilly, S., Bornovalova, M. & Trifunovic, R. Excitotoxic lesions of the gustatory thalamus spare simultaneous contrast effects but eliminate anticipatory negative contrast: evidence against a memory deficit. Behav. Neurosci. 118, 365–376 (2004).
Kullmann, S. et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum. Brain Mapp. 21 Apr 2011 (doi:10.1002/hbm.21268).
Stice, E., Spoor, S., Bohon, C., Veldhuizen, M. G. & Small, D. M. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J. Abnorm. Psychol. 117, 924–935 (2008).
Stice, E., Yokum, S., Burger, K. S., Epstein, L. H. & Small, D. M. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J. Neurosci. 31, 4360–4366 (2011). A key paper showing that intrinsic differences in brain signalling may predispose humans to obesity.
Wang, Z. et al. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J. Neurosci. 27, 14035–14040 (2007).
Naqvi, N. H., Rudrauf, D., Damasio, H. & Bechara, A. Damage to the insula disrupts addiction to cigarette smoking. Science 315, 531–534 (2007). An important paper suggesting that the insula may be involved in drug addiction.
Hollander, J. A., Lu, Q., Cameron, M. D., Kamenecka, T. M. & Kenny, P. J. Insular hypocretin transmission regulates nicotine reward. Proc. Natl Acad. Sci. USA 105, 19480–19485 (2008).
Contreras, M., Ceric, F. & Torrealba, F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 318, 655–658 (2007).
Unal, C. T., Beverley, J. A., Willuhn, I. & Steiner, H. Long-lasting dysregulation of gene expression in corticostriatal circuits after repeated cocaine treatment in adult rats: effects on zif 268 and homer 1a. Eur. J. Neurosci. 29, 1615–1626 (2009).
Schiltz, C. A., Bremer, Q. Z., Landry, C. F. & Kelley, A. E. Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biol. 5, 16 (2007).
Swank, M. W. & Sweatt, J. D. Increased histone acetyltransferase and lysine acetyltransferase activity and biphasic activation of the ERK/RSK cascade in insular cortex during novel taste learning. J. Neurosci. 21, 3383–3391 (2001).
Simonyi, A., Serfozo, P., Parker, K. E., Ramsey, A. K. & Schachtman, T. R. Metabotropic glutamate receptor 5 in conditioned taste aversion learning. Neurobiol. Learn. Mem. 92, 460–463 (2009).
Berman, D. E., Hazvi, S., Rosenblum, K., Seger, R. & Dudai, Y. Specific and differential activation of mitogen-activated protein kinase cascades by unfamiliar taste in the insular cortex of the behaving rat. J. Neurosci. 18, 10037–10044 (1998).
Rolls, E. T. Functional neuroimaging of umami taste: what makes umami pleasant? Am. J. Clin. Nutr. 90, 804S–813S (2009).
Morewedge, C. K., Huh, Y. E. & Vosgerau, J. Thought for food: imagined consumption reduces actual consumption. Science 330, 1530–1533 (2010). An intriguing finding suggesting that mental representations of consuming a particular food item may be sufficient to trigger satiety in the absence of actually eating the food item. The paper highlights the importance of higher-order cortical brain sites in regulating the relative incentive value of particular food items.
Salzman, C. D. & Fusi, S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu. Rev. Neurosci. 33, 173–202 (2010).
Volkow, N. D. et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42, 1537–1543 (2008). An important paper demonstrating that altered D2 receptor density in the striatum is associated with altered cortical activity in obese individuals, which may influence their ability to control food intake.
Woolley, J. D. et al. Binge eating is associated with right orbitofrontalinsularstriatal atrophy in frontotemporal dementia. Neurology 69, 1424–1433 (2007).
Mena, J. D., Sadeghian, K. & Baldo, B. A. Induction of hyperphagia and carbohydrate intake by mu-opioid receptor stimulation in circumscribed regions of frontal cortex. J. Neurosci. 31, 3249–3260 (2011).
Kantak, K. M., Mashhoon, Y., Silverman, D. N., Janes, A. C. & Goodrich, C. M. Role of the orbitofrontal cortex and dorsal striatum in regulating the dose-related effects of self-administered cocaine. Behav. Brain Res. 201, 128–136 (2009).
Burke, K. A., Franz, T. M., Miller, D. N. & Schoenbaum, G. The role of the orbitofrontal cortex in the pursuit of happiness and more specific rewards. Nature 454, 340–344 (2008).
Pears, A., Parkinson, J. A., Hopewell, L., Everitt, B. J. & Roberts, A. C. Lesions of the orbitofrontal but not medial prefrontal cortex disrupt conditioned reinforcement in primates. J. Neurosci. 23, 11189–11201 (2003).
Hutcheson, D. M. & Everitt, B. J. The effects of selective orbitofrontal cortex lesions on the acquisition and performance of cue-controlled cocaine seeking in rats. Ann. NY Acad. Sci. 1003, 410–411 (2003).
George, O., Mandyam, C. D., Wee, S. & Koob, G. F. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology 33, 2474–2482 (2008).
Homayoun, H. & Moghaddam, B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J. Neurosci. 26, 8025–8039 (2006).
Schoenbaum, G. & Shaham, Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol. Psychiatry 63, 256–262 (2008).
Winstanley, C. A. et al. ΔFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J. Neurosci. 27, 10497–10507 (2007).
Winstanley, C. A. et al. Increased impulsivity during withdrawal from cocaine self-administration: role for ΔFosB in the orbitofrontal cortex. Cereb. Cortex 19, 435–444 (2009). An elegant demonstration that adaptive responses in the OFC in response to drugs of abuse can impact complex behavioural states, which may in turn influence vulnerability to develop compulsive drug seeking behaviours.
Sclafani, A. Post-ingestive positive controls of ingestive behavior. Appetite 36, 79–83 (2001).
Ren, X. et al. Nutrient selection in the absence of taste receptor signaling. J. Neurosci. 30, 8012–8023 (2010).
de Araujo, I. E. et al. Food reward in the absence of taste receptor signaling. Neuron 57, 930–941 (2008). A seminal paper demonstrating that post-ingestive effects of palatable food, independent of their taste, can support food reward and drive preference for food that is high in macronutrients like fats and sugars.
Perez, C.A. et al. A transient receptor potential channel expressed in taste receptor cells. Nature Neurosci. 5, 1169–1176 (2002).
Oliveira-Maia, A. J. et al. Nicotine activates TRPM5-dependent and independent taste pathways. Proc. Natl Acad. Sci. USA 106, 1596–1601 (2009).
Blednov, Y. A. et al. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 7, 1–13 (2008).
Vucetic, Z. & Reyes, T. M. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 577–593 (2010).
Muller, D. L. & Unterwald, E. M. D1 dopamine receptors modulate ΔFosB induction in rat striatum after intermittent morphine administration. J. Pharmacol. Exp. Ther. 314, 148–154 (2005).
Nestler, E. J. Review. Transcriptional mechanisms of addiction: role of ΔFosB. Phil. Trans. R Soc. Lond. B 363, 3245–3255 (2008).
Teegarden, S. L., Scott, A. N. & Bale, T. L. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience 162, 924–932 (2009).
Christiansen, A. M., Dekloet, A. D., Ulrich-Lai, Y. M. & Herman, J. P. “Snacking” causes long term attenuation of HPA axis stress responses and enhancement of brain FosB/ΔFosB expression in rats. Physiol. Behav. 103, 111–116 (2011).
Wallace, D. L. et al. The influence of ΔFosB in the nucleus accumbens on natural reward-related behavior. J. Neurosci. 28, 10272–10277 (2008). This paper shows that a transcription factor that is implicated in addiction can also influence consumption of natural rewards like food.
Teegarden, S. L. & Bale, T. L. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol. Psychiatry 61, 1021–1029 (2007).
Stamp, J. A., Mashoodh, R., van Kampen, J. M. & Robertson, H. A. Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and ΔFosB expression in the nucleus accumbens of the rat. Brain Res. 1204, 94–101 (2008).
Olausson, P. et al. ΔFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J. Neurosci. 26, 9196–9204 (2006).
Colby, C. R., Whisler, K., Steffen, C., Nestler, E. J. & Self, D. W. Striatal cell type-specific overexpression of ΔFosB enhances incentive for cocaine. J. Neurosci. 23, 2488–2493 (2003).
Teegarden, S. L., Nestler, E. J. & Bale, T. L. Delta FosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol. Psychiatry 64, 941–950 (2008).
Bibb, J. A. et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature 410, 376–380 (2001).
Kumar, A. et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48, 303–314 (2005).
Taylor, J. R. et al. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proc. Natl Acad. Sci. USA 104, 4147–4152 (2007).
Benavides, D. R. et al. Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. J. Neurosci. 27, 12967–12976 (2007).
Gupta, A. & Tsai, L. H. Neuroscience. A kinase to dampen the effects of cocaine? Science 292, 236–237 (2001).
Stipanovich, A. et al. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature 453, 879–884 (2008).
Skofitsch, G., Jacobowitz, D. M. & Zamir, N. Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res. Bull. 15, 635–649 (1985).
de Lecea, L. et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl Acad. Sci. USA 95, 322–327 (1998).
Qu, D. et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 380, 243–247 (1996).
Hara, J. et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30, 345–354 (2001). An important paper showing that hypocretin transmission controls food intake.
Georgescu, D. et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J. Neurosci. 25, 2933–2940 (2005).
Sears, R. M. et al. Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. J. Neurosci. 30, 8263–8273 (2010).
Chung, S. et al. The melanin-concentrating hormone system modulates cocaine reward. Proc. Natl Acad. Sci. USA 106, 6772–6777 (2009).
Zheng, H., Patterson, L. M. & Berthoud, H. R. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J. Neurosci. 27, 11075–11082 (2007).
Uramura, K. et al. Orexina activates phospholipase C and protein kinase Cmediated Ca2+ signaling in dopamine neurons of the ventral tegmental area. Neuroreport 12, 1885–1889 (2001).
Cason, A. M. et al. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol. Behav. 100, 419–428 (2010).
Skibicka, K. P., Hansson, C., Alvarez-Crespo, M., Friberg, P. A. & Dickson, S. L. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience 180, 129–137 (2011).
Farooqi, I. S. et al. Leptin regulates striatal regions and human eating behavior. Science 317, 1355 (2007). An elegant demonstration that leptin can influence activity in brain reward systems and may thereby control food intake.
Figlewicz, D. P., Evans, S. B., Murphy, J., Hoen, M. & Baskin, D. G. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 964, 107–115 (2003).
Fulton, S. et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51, 811–822 (2006).
Hommel, J. D. et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51, 801–810 (2006).
Morton, G. J., Blevins, J. E., Kim, F., Matsen, M. & Figlewicz, D. P. The action of leptin in the ventral tegmental area to decrease food intake is dependent on Jak2 signaling. Am. J. Physiol. Endocrinol. Metab. 297, e202–e210 (2009).
Bruijnzeel, A. W., Corrie, L. W., Rogers, J. A. & Yamada, H. Effects of insulin and leptin in the ventral tegmental area and arcuate hypothalamic nucleus on food intake and brain reward function in female rats. Behav. Brain Res. 219, 254–264 (2011).
Davis, J. F. et al. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol. Psychiatry 69, 668–674 (2011).
Vaisse, C. et al. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nature Genet. 14, 95–97 (1996).
Berhow, M. T., Hiroi, N., Kobierski, L. A., Hyman, S. E. & Nestler, E. J. Influence of cocaine on the JAK-STAT pathway in the mesolimbic dopamine system. J. Neurosci. 16, 8019–8026 (1996).
Zahniser, N. R., Goens, M. B., Hanaway, P. J. & Vinych, J. V. Characterization and regulation of insulin receptors in rat brain. J. Neurochem. 42, 1354–1362 (1984).
Figlewicz, D. P., Bennett, J. L., Aliakbari, S., Zavosh, A. & Sipols, A. J. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R388–R394 (2008).
Konner, A. C. et al. Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell Metab. 13, 720–728 (2011).
Kamei, J. & Ohsawa, M. Effects of diabetes on methamphetamine-induced place preference in mice. Eur. J. Pharmacol. 318, 251–256 (1996).
Murzi, E. et al. Diabetes decreases limbic extracellular dopamine in rats. Neurosci. Lett. 202, 141–144 (1996).
Cordeira, J. W., Frank, L., Sena-Esteves, M., Pothos, E. N. & Rios, M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J. Neurosci. 30, 2533–2541 (2010).
Krugel, U., Schraft, T., Kittner, H., Kiess, W. & Illes, P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur. J. Pharmacol. 482, 185–187 (2003).
Roseberry, A. G., Painter, T., Mark, G. P. & Williams, J. T. Decreased vesicular somatodendritic dopamine stores in leptin-deficient mice. J. Neurosci. 27, 7021–7027 (2007).
Iniguez, S. D. et al. Insulin receptor substrate2 in the ventral tegmental area regulates behavioral responses to cocaine. Behav. Neurosci. 122, 1172–1177 (2008).
Russo, S. J. et al. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nature Neurosci. 10, 93–99 (2007).
Schoffelmeer, A. N. et al. Insulin modulates cocaine-sensitive monoamine transporter function and impulsive behavior. J. Neurosci. 31, 1284–1291 (2011).
Belin, D., Mar., A. C., Dalley, J. W., Robbins, T. W. & Everitt, B. J. High impulsivity predicts the switch to compulsive cocaine-taking. Science 320, 1352–1355 (2008).
Brewer, J. A. & Potenza, M. N. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem. Pharmacol. 75, 63–75 (2008).
Wang, X. et al. Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J. Neurosci. 30, 12632–12641 (2010).
Hou, L. & Klann, E. Activation of the phosphoinositide 3kinaseAkt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 24, 6352–6361 (2004).
Kasanetz, F. et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science 328, 1709–1712 (2010).
Brown, A. L., Flynn, J. R., Smith, D. W. & Dayas, C. V. Down-regulated striatal gene expression for synaptic plasticity-associated proteins in addiction and relapse vulnerable animals. Int. J. Neuropsychopharmacol. 14, 1099–1110 (2010).
Lafourcade, M. et al. Nutritional omega3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nature Neurosci. 14, 345–350 (2011). This paper shows that a fatty acid typically found in oily fish can influence endocannabinoid signalling — an important component of the brain reward systems.
Jiao, S. & Li, Z. Nonapoptotic function of BAD and BAX in long-term depression of synaptic transmission. Neuron 70, 758–772 (2011).
Li, Z. et al. Caspase3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell 141, 859–871 (2010).
Burguillos, M. A. et al. Caspase signalling controls microglia activation and neurotoxicity. Nature 472, 319–324 (2011).
Bishnoi, M., Chopra, K. & Kulkarni, S. K. Activation of striatal inflammatory mediators and caspase3 is central to haloperidol-induced orofacial dyskinesia. Eur. J. Pharmacol. 590, 241–245 (2008).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Zhang, X. et al. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008). A seminal paper showing that circulating inflammatory cytokines can impact hypothalamic function and thereby influence food intake.
Kleinridders, A. et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 10, 249–259 (2009).
Purkayastha, S., Zhang, G. & Cai, D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NFκB. Nature medicine 17, 883–887 (2011).
Cazettes, F., Cohen, J. I., Yau, P. L., Talbot, H. & Convit, A. Obesity-mediated inflammation may damage the brain circuit that regulates food intake. Brain Res. 1373, 101–109 (2011).
Russo, S. J. et al. Nuclear factor κ B signaling regulates neuronal morphology and cocaine reward. J. Neurosci. 29, 3529–3537 (2009). An important paper showing that inflammation in brain reward systems may contribute to drug addiction.
Ang, E. et al. Induction of nuclear factor-κB in nucleus accumbens by chronic cocaine administration. J. Neurochem. 79, 221–224 (2001).
Crews, F. T., Zou, J. & Qin, L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav. Immun. 25, S4–S12 (2011).
Yeung, F. et al. Modulation of NFκBdependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 (2004).
Ramadori, G. et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 12, 78–87 (2010).
Renthal, W. et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron 62, 335–348 (2009).
Turek, F. W. et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045 (2005).
McClung, C.A. et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc. Natl Acad. Sci. USA 102, 9377–9381 (2005).
Maas, S. Gene regulation through RNA editing. Discov. Med. 10, 379–386 (2010).
Burns, C. M. et al. Regulation of serotonin-2C receptor Gprotein coupling by RNA editing. Nature 387, 303–308 (1997).
Kishore, S. & Stamm, S. The snoRNA HBII52 regulates alternative splicing of the serotonin receptor 2C. Science 311, 230–232 (2006).
Sahoo, T. et al. Prader-Willi phenotype caused by paternal deficiency for the HBII85 C/D box small nucleolar RNA cluster. Nature Genet. 40, 719–721 (2008).
Hollander, J. A. et al. Striatal microRNA controls cocaine intake through CREB signalling. Nature 466, 197–202 (2010).
Ryan, K. K. et al. A role for central nervous system PPAR-γ in the regulation of energy balance. Nature Med. 17, 623–626 (2011).
Lu, M. et al. Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nature Med. 17, 618–622 (2011). This paper and also reference 156 show that PPARγ in brain may control food intake.
Stopponi, S. et al. Activation of nuclear PPARγ receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol. Psychiatry 69, 642–649 (2011).
Noonan, M. A., Bulin, S. E., Fuller, D. C. & Eisch, A. J. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J. Neurosci. 30, 304–315 (2010).
Yokoyama, T. K., Mochimaru, D., Murata, K., Manabe, H., Kobayakawa, K., Kobayakawa, R., Sakano, H., Mori, K., Yamaguchi, M. Elimination of adult-born neurons in the olfactory bulb is promoted during the postprandial period. Neuron 71, 883–897 (2011).
Mineur, Y. S. et al. Nicotine decreases food intake through activation of POMC neurons. Science 332, 1330–1332 (2011).
Church, C. et al. Overexpression of Fto leads to increased food intake and results in obesity. Nature Genet. 42, 1086–1092 (2010).
Vucetic, Z., Kimmel, J., Totoki, K., Hollenbeck, E. & Reyes, T. M. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 151, 4756–4764 (2010).
Vucetic, Z., Kimmel, J. & Reyes, T. M. Chronic high-fat diet drives postnatal epigenetic regulation of mu-opioid receptor in the brain. Neuropsychopharmacology 36, 1199–1206 (2011). A very important finding suggesting that alterations in DNA methylation can influence vulnerability to addiction.
Dunn, G. A. & Bale, T. L. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology 152, 2228–2236 (2011). This important paper suggests that diet can trigger epigenetic alterations that can influence dietary preference and be transmitted through generations.
Dallman, M. F. et al. Chronic stress and obesity: a new view of “comfort food”. Proc. Natl Acad. Sci. USA 100, 11696–11701 (2003).
Cottone, P. et al. CRF system recruitment mediates dark side of compulsive eating. Proc. Natl Acad. Sci. USA 106, 20016–20020 (2009).
Koob, G. F. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 1314, 3–14 (2010).
Macht, M. Effects of high- and low-energy meals on hunger, physiological processes and reactions to emotional stress. Appetite 26, 71–88 (1996).
Oswald, K. D., Murdaugh, D. L., King, V. L. & Boggiano, M. M. Motivation for palatable food despite consequences in an animal model of binge eating. Int. J. Eat Disord. 44, 203–211 (2010).
Hagan, M. M. et al. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol. Behav. 77, 45–54 (2002).
Acknowledgements
The author is supported by grants from the US National Institute on Drug Abuse (NIDA). This is manuscript number 21309 from The Scripps Research Institute.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Hyperphagia
-
Excessive consumption of food (above caloric requirements), which can reflect increased motivation to consume palatable food and/or deficits in brain circuitries that regulate satiety.
- Protracted drug abstinence
-
This is an aversive state that can persist in drug-dependent subjects long after cessation of drug use. Protracted abstinence is thought to increase vulnerability to relapse to drug-taking behaviour.
- Reinforcer
-
This is a stimulus (object or event) that is obtained or that occurs in response to a particular behaviour and that is associated with an increased probability that the behavioural response that resulted in delivery of the stimulus will occur again. In essence, a reinforcer is anything that increases the likelihood that a given behaviour will be repeated.
- Direct pathway
-
The direct striatal pathway comprises medium spiny neurons (MSNs) that express dopamine D1 receptors and project directly to the globus pallidus interna (GPi). The indirect pathway comprises MSNs that express dopamine D2 receptors and project to the GPi indirectly through a pathway involving the globus pallidus externa (GPe) and the subthalamic nucleus.
- Fixed and progressive ratio schedules
-
A fixed ratio schedule of reinforcement requires an animal to emit a fixed number of responses to earn a reinforcer. A progressive ratio schedule involves the animal emitting progressively greater numbers of responses to earn each subsequent reinforcer.
- Anorexigenic
-
A stimulus (object or event) that decreases appetite and food consumption.
Rights and permissions
About this article
Cite this article
Kenny, P. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci 12, 638–651 (2011). https://doi.org/10.1038/nrn3105
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3105
This article is cited by
-
The genetics of a “femaleness/maleness” score in cardiometabolic traits in the UK biobank
Scientific Reports (2023)
-
Central effects of opioidergic system on food intake in birds and mammals: a review
Veterinary Research Communications (2023)
-
Involvement of the ghrelin system in the maintenance and reinstatement of cocaine-motivated behaviors: a role of adrenergic action at peripheral β1 receptors
Neuropsychopharmacology (2022)
-
Involvement of the ghrelin system in the maintenance of oxycodone self-administration: converging evidence from endocrine, pharmacologic and transgenic approaches
Molecular Psychiatry (2022)
-
Diet-induced deficits in goal-directed control are rescued by agonism of group II metabotropic glutamate receptors in the dorsomedial striatum
Translational Psychiatry (2022)