Key Points
-
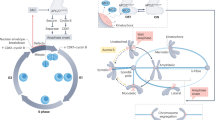
The major goal of mitosis is to distribute the genetic material equally between two daughter cells.
-
Movement of the chromosomes on the spindle is driven by both the polymerization dynamics of microtubules and the molecular motor proteins that associate with kinetochores and chromosome arms.
-
The dynamic linkage of the microtubules to the kinetochore is probably a major factor that drives chromosome movements.
-
Chromosome congression consists of multiple individual mechanisms that enhance the fidelity of mitosis.
-
A complete understanding of the mechanisms that drive chromosome motility requires an integrated approach, combining molecular and cell biology, high-resolution imaging, biophysical and biochemical reconstitution approaches, and mathematical and computational tools.
Abstract
For over a century, scientists have strived to understand the mechanisms that govern the accurate segregation of chromosomes during mitosis. The most intriguing feature of this process, which is particularly prominent in higher eukaryotes, is the complex behaviour exhibited by the chromosomes. This behaviour is based on specific and highly regulated interactions between the chromosomes and spindle microtubules. Recent discoveries, enabled by high-resolution imaging combined with the various genetic, molecular, cell biological and chemical tools, support the idea that establishing and controlling the dynamic interaction between chromosomes and microtubules is a major factor in genomic fidelity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sauer, G. et al. Proteome analysis of the human mitotic spindle. Mol. Cell. Proteomics 4, 35–43 (2005).
Musacchio, A. & Salmon, E. D. The spindle-assembly checkpoint in space and time. Nature Rev. Mol. Cell Biol. 8, 379–393 (2007).
Holland, A. J. & Cleveland, D. W. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nature Rev. Mol. Cell Biol. 10, 478–487 (2009).
Rajagopalan, H. & Lengauer, C. Aneuploidy and cancer. Nature 432, 338–341 (2004).
Kuriyama, R. & Borisy, G. G. Microtubule-nucleating activity of centrosomes in chinese hamster ovary cells is independent of the centriole cycle but coupled to the mitotic cycle. J. Cell Biol. 91, 822–826 (1981).
Saxton, W. M. et al. Tubulin dynamics in cultured mammalian cells. J. Cell Biol. 99, 2175–2186 (1984).
Walczak, C. E. & Heald, R. Mechanisms of mitotic spindle assembly and function. Int. Rev. Cytol. 265, 111–158 (2008).
Cheeseman, I. M. & Desai, A. Molecular architecture of the kinetochore-microtubule interface. Nature Rev. Mol. Cell Biol. 9, 33–46 (2008).
Santaguida, S. & Musacchio, A. The life and miracles of kinetochores. EMBO J. 28, 2511–2531 (2009).
Brinkley, B. R. & Stubblefield, E. The fine structure of the kinetochore of a mammalian cell in vitro. Chromosoma 19, 28–43 (1966).
Jokelainen, P. T. The ultrastructure and spatial organization of the metaphase kinetochore in mitotic rat cells. J. Ultrastruct. Res. 19, 19–44 (1967).
Ris, H. & Witt, P. L. Structure of the mammalian kinetochore. Chromosoma 82, 153–170 (1981).
McEwen, B. F., Hsieh, C. E., Mattheyses, A. L. & Rieder, C. L. A new look at kinetochore structure in vertebrate somatic cells using high-pressure freezing and freeze substitution. Chromosoma 107, 366–375 (1998).
Dong, Y., Vanden Beldt, K. J., Meng, X., Khodjakov, A. & McEwen, B. F. The outer plate in vertebrate kinetochores is a flexible network with multiple microtubule interactions. Nature Cell Biol. 9, 516–522 (2007). A high-resolution look at the ultrastructure of the kinetochore.
Vagnarelli, P., Ribeiro, S. A. & Earnshaw, W. C. Centromeres: old tales and new tools. FEBS Lett. 582, 1950–1959 (2008).
Cimini, D., Moree, B., Canman, J. C. & Salmon, E. D. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J. Cell Sci. 116, 4213–4225 (2003).
Ganem, N. J., Godinho, S. A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 (2009).
Silkworth, W. T., Nardi, I. K., Scholl, L. M. & Cimini, D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS One 4, e6564 (2009). References 17 and 18 make the important discovery that multipolarity leads to improper kinetochore attachments, which are the ultimate source of chromosome instability.
Thompson, S. L. & Compton, D. A. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 180, 665–672 (2008).
Nicklas, R. B., Krawitz, L. E. & Ward, S. C. Odd chromosome movement and inaccurate chromosome distribution in mitosis and meiosis after treatment with protein kinase inhibitors. J. Cell Sci. 104, 961–973 (1993).
Cimini, D., Wan, X., Hirel, C. B. & Salmon, E. D. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr. Biol. 16, 1711–1718 (2006).
DeLuca, J. G. et al. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127, 969–982 (2006).
Andrews, P. D. et al. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6, 253–268 (2004).
Lan, W. et al. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 14, 273–286 (2004).
Ohi, R., Sapra, T., Howard, J. & Mitchison, T. J. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol. Biol. Cell 15, 2895–2906 (2004).
Liu, D., Vader, G., Vromans, M. J., Lampson, M. A. & Lens, S. M. Sensing chromosome bi-orientation by spatial separation of Aurora B kinase from kinetochore substrates. Science 323, 1350–1353 (2009).
Nicklas, R. B. How cells get the right chromosomes. Science 275, 632–637 (1997).
Fuller, B. G. et al. Midzone activation of Aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 453, 1132–1136 (2008).
Bakhoum, S. F., Genovese, G. & Compton, D. A. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr. Biol. 19, 1937–1942 (2009).
Bakhoum, S. F., Thompson, S. L., Manning, A. L. & Compton, D. A. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nature Cell Biol. 11, 27–35 (2009).
Hill, T. L. Theoretical problems related to the attachment of microtubules to kinetochores. Proc. Natl. Acad. Sci. USA 82, 4404–4408 (1985).
Lombillo, V. A., Nislow, C., Yen, T. J., Gelfand, V. I. & McIntosh, J. R. Antibodies to the kinesin motor domain and CENPE inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J. Cell Biol. 128, 107–115 (1995).
Lombillo, V. A., Stewart, R. J. & McIntosh, J. R. Minus-end-directed motion of kinesin-coated microspheres driven by microtubule depolymerization. Nature 373, 161–164 (1995). An elegant demonstration of the insight provided by in vitro reconstitution experiments.
Martin-Lluesma, S., Stucke, V. M. & Nigg, E. A. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 297, 2267–2270 (2002).
DeLuca, J. G. et al. Nuf2 and Hec1 are required for retention of the checkpoint proteins Mad1 and Mad2 to kinetochores. Curr. Biol. 13, 2103–2109 (2003).
McCleland, M. L. et al. The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev. 17, 101–114 (2003).
Cheeseman, I. M., Chappie, J. S., Wilson-Kubalek, E. M. & Desai, A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127, 983–997 (2006).
Powers, A. F. et al. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell 136, 865–875 (2009).
Wilson-Kubalek, E. M., Cheeseman, I. M., Yoshioka, C., Desai, A. & Milligan, R. A. Orientation and structure of the Ndc80 complex on the microtubule lattice. J. Cell Biol. 182, 1055–1061 (2008).
Westermann, S. et al. Formation of a dynamic kinetochore–microtubule interface through assembly of the Dam1 ring complex. Mol. Cell 17, 277–290 (2005).
Westermann, S. et al. The Dam1 kinetochore ring complex moves processively on depolymerizing microtubule ends. Nature 440, 565–569 (2006).
Grishchuk, E. L., Molodtsov, M. I., Ataullakhanov, F. I. & McIntosh, J. R. Force production by disassembling microtubules. Nature 438, 384–388 (2005).
Grishchuk, E. L., Spiridonov, I. S. & McIntosh, J. R. Mitotic chromosome biorientation in fission yeast is enhanced by dynein and a minusenddirected, kinesin-like protein. Mol. Biol. Cell 18, 2216–2225 (2007).
Daum, J. R. et al. Ska3 Is required for spindle checkpoint silencing and the maintenance of chromosome cohesion in mitosis. Curr. Biol. 19, 1467–1472 (2009).
Gaitanos, T. N. et al. Stable kinetochore-microtubule interactions depend on the Ska complex and its new component Ska3/C13Orf3. EMBO J. 28, 1442–1452 (2009).
Raaijmakers, J. A., Tanenbaum, M. E., Maia, A. F. & Medema, R. H. RAMA1 is a novel kinetochore protein involved in kinetochore-microtubule attachment. J. Cell Sci. 122, 2436–2445 (2009).
Liu, X., McLeod, I., Anderson, S., Yates, J. R. 3rd, & He, X. Molecular analysis of kinetochore architecture in fission yeast. EMBO J. 24, 2919–2930 (2005).
Sanchez-Perez, I. et al. The DASH complex and Klp5/Klp6 kinesin coordinate bipolar chromosome attachment in fission yeast. EMBO J. 24, 2931–2943 (2005).
Peterson, J. B. & Ris, H. Electron-microscopic study of the spindle and chromosome movement in the yeast Saccharomyces cerevisiae. J. Cell Sci. 22, 219–242 (1976).
Winey, M. et al. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J. Cell Biol. 129, 1601–1615 (1995).
Grishchuk, E. L. et al. The Dam1 ring binds microtubules strongly enough to be a processive as well as energy-efficient coupler for chromosome motion. Proc. Natl. Acad. Sci. USA 105, 15423–15428 (2008).
McIntosh, J. R. et al. Fibrils connect microtubule tips with kinetochores: a mechanism to couple tubulin dynamics to chromosome motion. Cell 135, 322–333 (2008). A high-resolution look at the ultrastructure of the kinetochore–microtubule linkage.
McEwen, B. F., Heagle, A. B., Cassels, G. O., Buttle, K. F. & Rieder, C. L. Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset. J. Cell Biol. 137, 1567–1580 (1997).
VandenBeldt, K. J. et al. Kinetochores use a novel mechanism for coordinating the dynamics of individual microtubules. Curr. Biol. 16, 1217–1223 (2006). This paper reports the surprising finding that the distribution of microtubule-end states at the kinetochore are mixed on both leading and lagging kinetochores.
Mazia, D. in The Cell, Vol. 3 (eds. Brachet, J. & Mirsky, A. E.) 77–412 (Academic Press, Inc., New York, 1961).
Rieder, C. L., Davison, E. A., Jensen, L. C. W., Cassimeris, L. & Salmon, E. D. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster and the half-spindle. J. Cell Biol. 103, 581–591 (1986).
Khodjakov, A. & Rieder, C. L. Kinetochores moving away from their associated pole do not exert a significant pushing force on the chromosome. J. Cell Biol. 135, 315–327 (1996). A classic cytology paper defining forces on kinetochores.
Rieder, C. L. & Salmon, E. D. Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle. J. Cell Biol. 124, 223–233 (1994).
Ke, K., Cheng, J. & Hunt, A. J. The distribution of polar ejection forces determines the amplitude of chromosome directional instability. Curr. Biol. 19, 807–815 (2009).
Skibbens, R., Skeen, V. P. & Salmon, E. D. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: a push-pull mechanism. J. Cell Biol. 122, 859–875 (1993). The first paper to define the features of kinetochore directional instability.
Kapoor, T. M. & Compton, D. A. Searching for the middle ground: mechanisms of chromosome alignment during mitosis. J. Cell Biol. 157, 551–556 (2002).
Rieder, C. L. & Alexander, S. Kinetochores are transported polewards along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J. Cell Biol. 110, 81–95 (1990).
Cai, S., O'Connell, C. B., Khodjakov, A. & Walczak, C. E. Chromosome congression in the absence of kinetochore fibres. Nature Cell Biol. 11, 832–838 (2009).
Kapoor, T. M. et al. Chromosomes can congress to the metaphase plate before biorientation. Science 311, 388–391 (2006). This seminal paper redefined ideas on how chromosomes congress to the spindle equator.
Wignall, S. M. & Villeneuve, A. M. Lateral microtubule bundles promote chromosome alignment during acentrosomal oocyte meiosis. Nature Cell Biol. 11, 839–844 (2009).
Brunet, S. et al. Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J. Cell Biol. 146, 1–12 (1999).
Yang, Z., Tulu, U. S., Wadsworth, P. & Rieder, C. L. Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr. Biol. 17, 973–980 (2007).
Kirschner, M. W. & Mitchison, T. J. Beyond self assembly: from microtubules to morphogenesis. Cell 45, 329–342 (1986).
Schaar, B. T., Chan, G. K. T., Maddox, P., Salmon, E. D. & Yen, T. J. CENPE function at kinetochores is essential for chromosome alignment. J. Cell Biol. 139, 1373–1382 (1997).
Wood, K. W., Sakowicz, R., Goldstein, L. S. B. & Cleveland, D. W. CENPE is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell 91, 357–366 (1997).
Wollman, R. et al. Efficient chromosome capture requires a bias in the 'search-and-capture' process during mitotic-spindle assembly. Curr. Biol. 15, 828–832 (2005). An elegant example of how mathematical modelling can contribute to our understanding of chromosome motility.
Schrader, F. Mitosis (Columbia University Press, 1953).
Telzer, B. R., Moses, M. J. & Rosenbaum, J. L. Assembly of microtubules onto kinetochores of isolated mitotic chromosomes of HeLa cells. Proc. Natl. Acad. Sci. USA 72, 4023–7402 (1975).
Debrabander, M. et al. Microtubule dynamics during the cell cycle: the effects of taxol and nocodazole on the microtubule system of PtK2 cells at different stages of the mitotic cycle. Int. Rev. Cytology 101, 215–273 (1986).
Witt, P. L., Ris, H. & Borisy, G. G. Origin of kinetochore microtubules in Chinese hamster ovary cells. Chromosoma 81, 483–505 (1980).
Khodjakov, A., Copenagle, L., Gordon, M. B., Compton, D. A. & Kapoor, T. M. Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. J. Cell Biol. 160, 671–683 (2003). This paper was the first to truly challenge the search and capture model of chromosome congression.
Maiato, H., Rieder, C. L. & Khodjakov, A. Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis. J. Cell Biol. 167, 831–840 (2004).
Tulu, U. S., Fagerstrom, C., Ferenz, N. P. & Wadsworth, P. Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr. Biol. 16, 536–541 (2006).
Lambert, A. M. & Lloyd, C. W. in Microtubules (eds. Hyams, J. S. & Lloyd, C. W.) 325–342 (Wiley-Liss, New York, 1994).
McKim, K. S. & Hawley, R. S. Chromosomal control of meiotic cell division. Science 270, 1595–1601 (1995).
Ostergren, G. The mechanism of coordination in bivalents and multivalents. The theory of orientation by pulling. Hereditas 37, 85–156 (1951).
Hays, T. & Salmon, E. D. Poleward force at the kinetochore in metaphase depends on the number of kinetochore microtubules. J. Cell Biol. 110, 391–404 (1990).
Hays, T. S., Wise, D. & Salmon, E. D. Traction force on a kinetochore at metaphase acts as a linear function of kinetochore fiber length. J. Cell Biol. 93, 374–389 (1982).
Pfarr, C. M. et al. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature 345, 263–265 (1990).
Steuer, E. R., Wordeman, L., Schroer, T. A. & Sheetz, M. P. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature 345, 266–268 (1990).
Yen, T. J. et al. CENPE, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO J. 10, 1245–1254 (1991).
Hyman, A. A. & Mitchison, T. J. Two different microtubule-based motor activities with opposite polarites in kinetochores. Nature 351, 206–211 (1991).
Darlington, C. Recent Advances in Cytology. (London: Churchill, 1937).
Ault, J. G., DeMarco, A. J., Salmon, E. D. & Rieder, C. L. Studies on the ejection properties of asters: astral microtubule turnover influences the oscillatory behavior and positioning of mono-oriented chromosomes. J. Cell Sci. 99, 701–710 (1991). The first paper to clearly show the polar ejection force.
Tokai, N. et al. Kid, a novel kinesin-like DNA binding protein, is localized to chromosomes and the mitotic spindle. EMBO J. 15, 457–467 (1996).
Yajima, J. et al. The human chromokinesin Kid is a plus end-directed microtubule-based motor. EMBO J. 22, 1067–1074 (2003).
Antonio, C. et al. Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell 102, 425–435 (2000).
Funabiki, H. & Murray, A. W. The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell 102, 411–424 (2000).
Levesque, A. A. & Compton, D. A. The chromokinesin Kid is necessary for chromosome arm orientation and oscillation, but not congression, on mitotic spindles. J. Cell Biol. 154, 1135–1146 (2001).
Tokai-Nishizumi, N., Ohsugi, M., Suzuki, E. & Yamamoto, T. The chromokinesin Kid is required for maintenance of proper metaphase spindle size. Mol. Biol. Cell 16, 5455–5463 (2005).
Vernos, I. et al. Xklp1, a chromosomal Xenopus kinesin-like protein essential for spindle organization and chromosome positioning. Cell 81, 117–127 (1995).
Walczak, C. E., Vernos, I., Mitchison, T. J., Karsenti, E. & Heald, R. A model for the proposed roles of different microtubule based motor proteins in establishing spindle bipolarity. Curr. Biol. 8, 903–913 (1998).
Bringmann, H. et al. A kinesin-like motor inhibits microtubule dynamic instability. Science 303, 1519–1522 (2004).
Mazumdar, M., Sundareshan, S. & Misteli, T. Human chromokinesin KIF4A functions in chromosome condensation and segregation. J. Cell Biol. 166, 613–620 (2004).
Piehl, M. & Cassimeris, L. Organization and dynamics of growing microtubule plus ends during early mitosis. Mol. Biol. Cell 14, 916–925 (2003).
Rusan, N. M., Fagerstrom, C. J., Yvon, A. M. & Wadsworth, P. Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-α tubulin. Mol. Biol. Cell 12, 971–980 (2001).
Kim, Y., Heuser, J. E., Waterman, C. M. & Cleveland, D. W. CENPE combines a slow, processive motor and a flexible coiled coil to produce an essential motile kinetochore tether. J. Cell Biol. 181, 411–419 (2008).
McEwen, B. F. et al. CENPE is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol. Biol. Cell 12, 2289–2776 (2001).
Skibbens, R., Rieder, C. L. & Salmon, E. D. Kinetochore motility after severing between sister chromosomes using laser microsurgery: evidence that kinetochore directional instability and position is regulated by tension. J. Cell Sci. 108, 2537–2548 (1995).
Rieder, C. L., Davison, E. A., Jensen, L. C., Cassimeris, L. & Salmon, E. D. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster and half-spindle. J. Cell Biol. 103, 581–591 (1986).
Stumpff, J., von Dassow, G., Wagenbach, M., Asbury, C. & Wordeman, L. The kinesin-8 motor Kif18A suppresses kinetochore movements to control mitotic chromosome alignment. Dev. Cell 14, 252–262 (2008).
Gupta, M. L., Jr ., Carvalho, P., Roof, D. M. & Pellman, D. Plus end-specific depolymerase activity of Kip3, a kinesin8 protein, explains its role in positioning the yeast mitotic spindle. Nature Cell Biol. 8, 913–923 (2006).
Varga, V. et al. Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nature Cell Biol. 8, 957–962 (2006).
DeLuca, J. G. et al. Hec1 and Nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol. Biol. Cell 16, 519–531 (2005).
Joglekar, A. P. & Hunt, A. J. A simple, mechanistic model for directional instability during mitotic chromosome movements. Biophys. J. 83, 42–58 (2002).
Liu, J., Desai, A., Onuchic, J. N. & Hwa, T. An integrated mechanobiochemical feedback mechanism describes chromosome motility from prometaphase to anaphase in mitosis. Proc. Natl. Acad. Sci. USA 105, 13752–13757 (2008).
Civelekoglu-Scholey, G., Sharp, D. J., Mogilner, A. & Scholey, J. M. Model of chromosome motility in Drosophila embryos: adaptation of a general mechanism for rapid mitosis. Biophys. J. 90, 3966–3982 (2006).
Mogilner, A., Wollman, R., Civelekoglu-Scholey, G. & Scholey, J. Modeling mitosis. Trends Cell Biol. 16, 88–96 (2006).
Wordeman, L., Wagenbach, M. & von Dassow, G. MCAK facilitates chromosome movement by promoting kinetochore microtubule turnover. J. Cell Biol. 179, 789–869 (2007).
Wordeman, L. & Mitchison, T. J. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J. Cell Biol. 128, 95–104 (1995).
Kline-Smith, S. L., Khodjakov, A., Hergert, P. & Walczak, C. E. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol. Biol. Cell 15, 1146–1159 (2004).
Rizk, R. S. et al. MCAK and paclitaxel have differential effects on spindle microtubule organization and dynamics. Mol. Biol. Cell 20, 1639–165 (2009).
Khodjakov, A., Cole, R. W., McEwen, B. F., Buttle, K. F. & Rieder, C. L. Chromosome fragments possessing only one kinetochore can congress to the spindle equator. J. Cell Biol. 136, 229–240 (1997).
O'Connell, C. B., Loncarek, J., Kalab, P. & Khodjakov, A. Relative contributions of chromatin and kinetochores to mitotic spindle assembly. J. Cell Biol. 187, 43–51 (2009).
Wise, D. A. & Brinkley, B. R. Mitosis in cells with unreplicated genomes (MUGs): spindle assembly and behavior of centromere fragments. Cell. Motil. Cytoskeleton 36, 291–302 (1997).
Gorbsky, G. J., Sammak, P. J. & Borisy, G. G. Chromosomes move poleward in anaphase along stationary microtubules that coordinately disassemble from their kinetochore ends. J. Cell Biol. 104, 9–18 (1987).
Brust-Mascher, I. & Scholey, J. M. Microtubule flux and sliding in mitotic spindles of Drosophila embryos. Mol. Biol. Cell 13, 3967–3975 (2002).
Desai, A., Maddox, P. S., Mitchison, T. J. & Salmon, E. D. Anaphase A chromosome movement and poleward spindle microtubule flux occur at similar rates in Xenopus extract spindles. J. Cell Biol. 141, 703–713 (1998).
Maddox, P., Desai, A., Oegema, K., Mitchison, T. J. & Salmon, E. D. Poleward microtubule flux is a major component of spindle dynamics and anaphase a in mitotic Drosophila embryos. Curr. Biol. 12, 1670–1674 (2002).
Nicklas, R. B. The motor for poleward chromosome movement in anaphase is in or near the kinetochore. J. Cell Biol. 109, 2245–2255 (1989).
Echeverri, C. J., Paschal, B. M., Vaughan, K. T. & Vallee, R. B. Molecular characterization of the 50kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132, 617–633 (1996).
Goshima, G., Nedelec, F. & Vale, R. D. Mechanisms for focusing mitotic spindle poles by minus end-directed motor proteins. J. Cell Biol. 171, 229–240 (2005).
Sharp, D. J., Rogers, G. C. & Scholey, J. M. Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos. Nature Cell Biol. 2, 922–930 (2000).
Vorozhko, V. V., Emanuele, M. J., Kallio, M. J., Stukenberg, P. T. & Gorbsky, G. J. Multiple mechanisms of chromosome movement in vertebrate cells mediated through the Ndc80 complex and dynein/dynactin. Chromosoma 117, 169–179 (2008).
Maney, T., Hunter, A. W., Wagenbach, M. & Wordeman, L. Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J. Cell Biol. 142, 787–801 (1998).
Rogers, G. C. et al. Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature 427, 364–370 (2004).
Buster, D. W., Zhang, D. & Sharp, D. J. Poleward tubulin flux in spindles: regulation and function in mitotic cells. Mol. Biol. Cell 18, 3094–3104 (2007).
Ganem, N. J., Upton, K. & Compton, D. A. Efficient mitosis in human cells lacking poleward microtubule flux. Curr. Biol. 15, 1827–1832 (2005).
Gordon, M. B., Howard, L. & Compton, D. A. Chromosome movement in mitosis requires microtubule anchorage at spindle poles. J. Cell Biol. 152, 425–434 (2001).
Acknowledgements
The authors would like to thank all members of their laboratories for insightful discussions on chromosome motility. In particular, we are grateful to L. Weaver and J. Powers for their comments on the manuscript. Our research is supported by funding from the National Institutes of Health (GM59618 to C.E.W and GM59363 to A.K).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Glossary
- Centromere
-
A specialized thin region, often referred to as the 'primary constriction', on a chromosome. It is composed of highly condensed heterochromatin that serves as the platform for the assembly of kinetochores.
- Kinetochore
-
A structure that assembles on the mitotic chromosome to attach to microtubules of the spindle.
- K-fibre
-
A bundle of microtubules that connects to a kinetochore on a mitotic chromosome.
- Segregation
-
The process by which sister chromatids are distributed to two daughter cells.
- Spindle assembly checkpoint
-
A surveillance mechanism that monitors the attachment state of individual chromosomes. The checkpoint blocks anaphase onset until the kinetochores are attached to microtubules of the spindle.
- Centrosome
-
A specialized organelle at the spindle pole that is the site of microtubule nucleation.
- Congression
-
The process by which chromosomes align at the spindle equator.
- Bi-oriented
-
Here, used to describe the attachment of a chromosome to microtubules emanating from opposite spindle poles.
- Centromeric heterochromatin
-
Distinct regions of chromatin at the centromere of a chromosome that are highly condensed and contribute to specifying the location of kinetochore assembly.
- Astral microtubule
-
A microtubule that emanates from the spindle pole to the cell cortex.
- Mono-oriented
-
Here, used to describe the attachment of a chromosome to microtubules coming from a single spindle pole.
Rights and permissions
About this article
Cite this article
Walczak, C., Cai, S. & Khodjakov, A. Mechanisms of chromosome behaviour during mitosis. Nat Rev Mol Cell Biol 11, 91–102 (2010). https://doi.org/10.1038/nrm2832
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2832
This article is cited by
-
The Mis6 inner kinetochore subcomplex maintains CENP-A nucleosomes against centromeric non-coding transcription during mitosis
Communications Biology (2022)
-
Forcing dividing cancer cells to die; low‐dose drug combinations to prevent spindle pole clustering
Apoptosis (2021)
-
Wip1 controls the translocation of the chromosomal passenger complex to the central spindle for faithful mitotic exit
Cellular and Molecular Life Sciences (2021)
-
Targeting the IL-1β/EHD1/TUBB3 axis overcomes resistance to EGFR-TKI in NSCLC
Oncogene (2020)
-
Membrane and organelle dynamics during cell division
Nature Reviews Molecular Cell Biology (2020)