Abstract
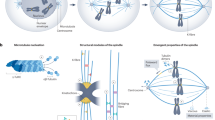
The transmission of a complete set of chromosomes to daughter cells during cell division is vital for development and tissue homeostasis. The spindle assembly checkpoint (SAC) ensures correct segregation by informing the cell cycle machinery of potential errors in the interactions of chromosomes with spindle microtubules prior to anaphase. To do so, the SAC monitors microtubule engagement by specialized structures known as kinetochores and integrates local mechanical and chemical cues such that it can signal in a sensitive, responsive and robust manner. In this Review, we discuss how SAC proteins interact to allow production of the mitotic checkpoint complex (MCC) that halts anaphase progression by inhibiting the anaphase-promoting complex/cyclosome (APC/C). We highlight recent advances aimed at understanding the dynamic signalling properties of the SAC and how it interprets various naturally occurring intermediate attachment states. Further, we discuss SAC signalling in the context of the mammalian multisite kinetochore and address the impact of the fibrous corona. We also identify current challenges in understanding how the SAC ensures high-fidelity chromosome segregation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cheeseman, I. M. The kinetochore. Cold Spring Harb. Perspect. Biol. 6, a015826 (2014).
McAinsh, A. D. & Marston, A. L. The four causes: the functional architecture of centromeres and kinetochores. Annu. Rev. Genet. 56, 279–314 (2022).
Musacchio, A. The molecular biology of spindle assembly checkpoint signaling dynamics. Curr. Biol. 25, R1002–R1018 (2015).
Lara-Gonzalez, P., Pines, J. & Desai, A. Spindle assembly checkpoint activation and silencing at kinetochores. Semin. Cell Dev. Biol. 117, 86–98 (2021).
London, N. & Biggins, S. Signalling dynamics in the spindle checkpoint response. Nat. Rev. Mol. Cell Biol. 15, 736–748 (2014).
Suzuki, A., Badger, B. L. & Salmon, E. D. A quantitative description of Ndc80 complex linkage to human kinetochores. Nat. Commun. 6, 8161 (2015).
Kiewisz, R. et al. Three-dimensional structure of kinetochore-fibers in human mitotic spindles. eLife 11, e75459 (2022). This study is a technical feat of 3D spindle reconstructions by electron tomography giving a unique view of all microtubules in human mitotic spindles.
Rago, F. & Cheeseman, I. M. Review series: The functions and consequences of force at kinetochores. J. Cell Biol. 200, 557–565 (2013).
Pines, J. Cubism and the cell cycle: the many faces of the APC/C. Nat. Rev. Mol. Cell Biol. 12, 427–438 (2011).
Barford, D. Structural interconversions of the anaphase-promoting complex/cyclosome (APC/C) regulate cell cycle transitions. Curr. Opin. Struct. Biol. 61, 86–97 (2020).
Sacristan, C. & Kops, G. J. P. L. Joined at the hip: kinetochores, microtubules, and spindle assembly checkpoint signaling. Trends Cell Biol. 25, 21–28 (2015).
Foley, E. A. & Kapoor, T. M. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat. Rev. Mol. Cell Biol. 14, 25–37 (2013).
Tanaka, T. U. Bi-orienting chromosomes: acrobatics on the mitotic spindle. Chromosoma 117, 521–533 (2008).
Collin, P., Nashchekina, O., Walker, R. & Pines, J. The spindle assembly checkpoint works like a rheostat rather than a toggle switch. Nat. Cell Biol. 15, 1378–1385 (2013).
Dick, A. E. & Gerlich, D. W. Kinetic framework of spindle assembly checkpoint signalling. Nat. Cell Biol. 15, 1370–1377 (2013).
Kops, G. J. P. L., Weaver, B. A. A. & Cleveland, D. W. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer 5, 773–785 (2005).
Ben-David, U. & Amon, A. Context is everything: aneuploidy in cancer. Nat. Rev. Genet. 21, 44–62 (2020).
Cohen, J. Sorting out chromosome errors. Science 296, 2164–2166 (2002).
McCoy, R. C. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet 33, 448–463 (2017).
Vázquez-Diez, C., Paim, L. M. G. & FitzHarris, G. Cell-size-independent spindle checkpoint failure underlies chromosome segregation error in mouse embryos. Curr. Biol. 29, 865–873.e3 (2019).
Currie, C. E. et al. The first mitotic division of human embryos is highly error prone. Nat. Commun. 13, 6755 (2022).
Hoevenaar, W. H. M. et al. Degree and site of chromosomal instability define its oncogenic potential. Nat. Commun. 11, 1501 (2020).
Trakala, M. et al. Clonal selection of stable aneuploidies in progenitor cells drives high-prevalence tumorigenesis. Genes Dev. 35, 1079–1092 (2021).
Foijer, F., Draviam, V. M. & Sorger, P. K. Studying chromosome instability in the mouse. Biochim. Biophys. Acta 1786, 73–82 (2008).
Hanks, S. et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat. Genet. 36, 1159–1161 (2004).
Yost, S. et al. Biallelic TRIP13 mutations predispose to Wilms tumor and chromosome missegregation. Nat. Genet. 49, 1148–1151 (2017).
Kuijt, T. E. F. et al. A biosensor for the mitotic kinase MPS1 reveal spatiotemporal activity dynamics and regulation. Curr. Biol. 30, 3862–3870 (2020).
Tighe, A., Johnson, V. L., Albertella, M. & Taylor, S. S. Aneuploid colon cancer cells have a robust spindle checkpoint. EMBO Rep. 2, 609–614 (2001).
Thompson, S. L. & Compton, D. A. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 180, 665–672 (2008).
Janssen, A., Kops, G. J. & Medema, R. H. Targeting the mitotic checkpoint to kill tumor cells. Horm. Cancer 2, 113–116 (2010).
Kops, G. J. P. L., Foltz, D. R. & Cleveland, D. W. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc. Natl Acad. Sci. USA 101, 8699–8704 (2004).
Cohen-Sharir, Y. et al. Aneuploidy renders cancer cells vulnerable to mitotic checkpoint inhibition. Nature 590, 486–491 (2021).
Kops, G. J. P. L., Snel, B. & Tromer, E. C. Evolutionary dynamics of the spindle assembly checkpoint in eukaryotes. Curr. Biol. 30, R589–R602 (2020).
Sudakin, V. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154, 925–936 (2001).
Hardwick, K. G., Johnston, R. C., Smith, D. L. & Murray, A. W. MAD3 encodes a novel component of the spindle checkpoint which interacts with Bub3p, Cdc20p, and Mad2p. J. Cell Biol. 148, 871–882 (2000).
Alfieri, C. et al. Molecular basis of APC/C regulation by the spindle assembly checkpoint. Nature 536, 431–436 (2016). This study presents the atomic structure of APC/CMCC and gives unprecedented insight into how the APC/C is inhibited by the SAC and how APC15 can promote MCC disassembly.
Izawa, D. & Pines, J. The mitotic checkpoint complex binds a second CDC20 to inhibit active APC/C. Nature 517, 631–634 (2015).
Rodriguez-Bravo, V. et al. Nuclear pores protect genome integrity by assembling a premitotic and Mad1-dependent anaphase inhibitor. Cell 156, 1017–1031 (2014).
Pachis, S. T. & Kops, G. J. P. L. Leader of the SAC: molecular mechanisms of Mps1/TTK regulation in mitosis. Open Biol. 8, 180109 (2018).
Maciejowski, J. et al. Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J. Cell Biol. 190, 89–100 (2010).
Hiruma, Y. et al. Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling. Science 348, 1264–1267 (2015).
Ji, Z., Gao, H. & Yu, H. Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. Science 348, 1260–1264 (2015). Together with Hiruma et al. (2015), this work illustrates the concept of mutually exclusive binding of MPS1 and microtubules to NDC80, providing a simple model for SAC silencing at kinetochores upon microtubule attachment.
Kemmler, S. et al. Mimicking Ndc80 phosphorylation triggers spindle assembly checkpoint signalling. EMBO J. 28, 1099–1110 (2009).
Gui, P. et al. Mps1 dimerization and multisite interactions with Ndc80 complex enable responsive spindle assembly checkpoint signaling. J. Mol. Cell Biol. 12, 486–498 (2020).
London, N., Ceto, S., Ranish, J. A. & Biggins, S. Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr. Biol. 22, 900–906 (2012).
Shepperd, L. A. et al. Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr. Biol. 22, 891–899 (2012).
Yamagishi, Y., Yang, C.-H., Tanno, Y. & Watanabe, Y. MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nat. Cell Biol. 14, 746–752 (2012).
Primorac, I. et al. Bub3 reads phosphorylated MELT repeats to promote spindle assembly checkpoint signaling. eLife 2, e01030 (2013).
Di Fiore, B. et al. The ABBA motif binds APC/C activators and is shared by APC/C substrates and regulators. Dev. Cell 32, 358–372 (2015).
Suijkerbuijk, S. J. E. et al. The vertebrate mitotic checkpoint protein BUBR1 is an unusual pseudokinase. Dev. Cell 22, 1321–1329 (2012).
Tromer, E., Bade, D., Snel, B. & Kops, G. J. P. L. Phylogenomics-guided discovery of a novel conserved cassette of short linear motifs in BubR1 essential for the spindle checkpoint. Open Biol. 6, 160315 (2016).
Overlack, K. et al. A molecular basis for the differential roles of Bub1 and BubR1 in the spindle assembly checkpoint. eLife 4, e05269 (2015).
Di Fiore, B., Wurzenberger, C., Davey, N. & Pines, J. The mitotic checkpoint complex requires an evolutionary conserved cassette to bind and inhibit active APC/C. Mol. Cell 64, 1144–1153 (2016).
Lischetti, T., Zhang, G., Sedgwick, G. G., Bolanos-garcia, V. M. & Nilsson, J. The internal Cdc20 binding site in BubR1 facilitates both spindle assembly checkpoint signalling and silencing. Nat. Commun. 5, 5563 (2014).
King, E. M. J., van der Sar, S. J. A. & Hardwick, K. G. Mad3 KEN boxes mediate both Cdc20 and Mad3 turnover, and are critical for the spindle checkpoint. PLoS ONE 2, e342 (2007).
Burton, J. L. & Solomon, M. J. Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint. Genes Dev. 21, 655–667 (2007).
Davenport, J., Harris, L. D. & Goorha, R. Spindle checkpoint function requires Mad2-dependent Cdc20 binding to the Mad3 homology domain of BubR1. Exp. Cell Res. 312, 1831–1842 (2006).
London, N. & Biggins, S. Mad1 kinetochore recruitment by Mps1-mediated phosphorylation of Bub1 signals the spindle checkpoint. Genes Dev. 28, 140–152 (2014).
Ji, Z., Gao, H., Jia, L., Li, B. & Yu, H. A sequential multi-target Mps1 phosphorylation cascade promotes spindle checkpoint signaling. eLife 6, e22513 (2017). This study shows how a cascade of phosphorylation events by MPS1 ensures recruitment of all necessary factors for MCC assembly.
Zhang, G. et al. Bub1 positions Mad1 close to KNL1 MELT repeats to promote checkpoint signalling. Nat. Commun. 8, 15822 (2017).
Klebig, C., Korinth, D. & Meraldi, P. Bub1 regulates chromosome segregation in a kinetochore-independent manner. J. Cell Biol. 185, 841–858 (2009).
Fischer, E. S. et al. Molecular mechanism of Mad1 kinetochore targeting by phosphorylated Bub1. EMBO Rep. 22, e52242 (2021).
Qian, J. et al. An attachment-independent biochemical timer of the spindle assembly checkpoint. Mol. Cell 68, e5 (2017).
Mapelli, M. & Musacchio, A. MAD contortions: conformational dimerization boosts spindle checkpoint signaling. Curr. Opin. Struct. Biol. 17, 716–725 (2007).
Mapelli, M., Massimiliano, L., Santaguida, S. & Musacchio, A. The Mad2 conformational dimer: structure and implications for the spindle assembly checkpoint. Cell 131, 730–743 (2007).
De Antoni, A. et al. The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr. Biol. 15, 214–225 (2005).
Piano, V. et al. CDC20 assists its catalytic incorporation in the mitotic checkpoint complex. Science 371, 67–71 (2021).
Lara-Gonzalez, P., Kim, T., Oegema, K., Corbett, K. & Desai, A. A tripartite mechanism catalyzes Mad2-Cdc20 assembly at unattached kinetochores. Science 67, 64–67 (2021).
Fischer, E. S. et al. Juxtaposition of Bub1 and Cdc20 on phosphorylated Mad1 during catalytic mitotic checkpoint complex assembly. Nat. Commun. 13, 6381 (2022). Together with Piano et al. (2021) and Lara-Gonzalez et al. (2021), this work shows how the MCC assembly catalysts (MPS1, MAD1–MAD2 and BUB1) ensure the proper spatial organization of components to catalyse MCC formation. Furthermore, Piano et al. (2021) show that this reaction is assisted by the CDC20 substrate.
Faesen, A. C. et al. Basis of catalytic assembly of the mitotic checkpoint complex. Nature 542, 498–502 (2017).
Simonetta, M. et al. The influence of catalysis on Mad2 activation dynamics. PLoS Biol. 7, e10 (2009).
Kops, G. J. P. L. & Gassmann, R. Crowning the kinetochore: the fibrous corona in chromosome segregation. Trends Cell Biol. 30, 653–667 (2020).
Sacristan, C. et al. Dynamic kinetochore size regulation promotes microtubule capture and chromosome biorientation in mitosis. Nat. Cell Biol. 20, 800–810 (2018).
Pereira, C. et al. Self-assembly of the RZZ complex into filaments drives kinetochore expansion in the absence of microtubule attachment. Curr. Biol. 28, 3408–3421.e8 (2018).
Raisch, T. et al. Structure of the RZZ complex and molecular basis of Spindly-driven corona assembly at human kinetochores. EMBO J. 41, e110411 (2022).
Rodriguez-Rodriguez, J. A. et al. Distinct roles of RZZ and Bub1-KNL1 in mitotic checkpoint signaling and kinetochore expansion. Curr. Biol. 28, 3422–3429.e5 (2018).
Silio, V., McAinsh, A. D. & Millar, J. B. KNL1-Bubs and RZZ provide two separable pathways for checkpoint activation at human kinetochores. Dev. Cell 35, 600–613 (2015).
Allan, L. A. et al. Cyclin B1 scaffolds MAD1 at the kinetochore corona to activate the mitotic checkpoint. EMBO J. 39, e103180 (2020). This study reports a unique recruiting mechanism of MAD1 to the fibrous corona through interaction with cyclin B1, which increases SAC signalling strength.
Jackman, M. et al. Cyclin B1–Cdk1 facilitates MAD1 release from the nuclear pore to ensure a robust spindle checkpoint. J. Cell Biol. 219, e201907082 (2020). This study shows that cyclin B promotes the SAC by ensuring MAD1 release from nuclear pore complexes and docking onto kinetochores.
Alfonso-Pérez, T., Hayward, D., Holder, J., Gruneberg, U. & Barr, F. A. MAD1-dependent recruitment of CDK1–CCNB1 to kinetochores promotes spindle checkpoint signaling. J. Cell Biol. 2018, 1108–1117 (2019). This study shows that cyclin B is recruited to kinetochores by interaction with MAD1, where it promotes a sustained SAC signal by enhancing recruitment of MPS1. Together with Allan et al. (2020) and Jackman et al. (2020), this paper shows a role for cyclin B in the SAC.
Hoyt, M. A., Totis, L. & Roberts, B. T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66, 507–517 (1991).
Bernard, P., Hardwick, K. & Javerzat, J. P. Fission yeast bub1 is a mitotic centromere protein essential for the spindle checkpoint and the preservation of correct ploidy through mitosis. J. Cell Biol. 143, 1775–1787 (1998).
Currie, C. E., Mora-Santos, M., Smith, C. A., McAinsh, A. D. & Millar, J. B. A. Bub1 is not essential for the checkpoint response to unattached kinetochores in diploid human cells. Curr. Biol. 28, R929–R930 (2018).
Meraldi, P. Bub1—the zombie protein that CRISPR cannot kill. EMBO J. 38, e101912 (2019).
Raaijmakers, J. A. et al. BUB1 is essential for the viability of human cells in which the spindle assembly checkpoint is compromised. Cell Rep. 22, 1424–1438 (2018).
Nilsson, J., Yekezare, M., Minshull, J. & Pines, J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat. Cell Biol. 10, 1411–1420 (2008).
Varetti, G., Guida, C., Santaguida, S., Chiroli, E. & Musacchio, A. Homeostatic control of mitotic arrest. Mol. Cell 44, 710–720 (2011).
Mansfeld, J., Collin, P., Collins, M. O., Choudhary, J. S. & Pines, J. APC15 drives the turnover of MCC–CDC20 to make the spindle assembly checkpoint responsive to kinetochore attachment. Nat. Cell Biol. 13, 1234–1243 (2011).
Foster, S. A. & Morgan, D. O. The APC/C subunit Mnd2/Apc15 promotes Cdc20 autoubiquitination and spindle assembly checkpoint inactivation. Mol. Cell 47, 921–932 (2012).
Uzunova, K. et al. APC15 mediates CDC20 autoubiquitylation by APC/CMCC and disassembly of the mitotic checkpoint complex. Nat. Struct. Mol. Biol. 19, 1116–1123 (2012).
Yamaguchi, M. et al. Cryo-EM of mitotic checkpoint complex-bound APC/C reveals reciprocal and conformational regulation of ubiquitin ligation. Mol. Cell 63, 593–607 (2016).
Gu, Y., Desai, A. & Corbett, K. D. Evolutionary dynamics and molecular mechanisms of HORMA domain protein signaling. Annu. Rev. Biochem. 91, 541–569 (2022).
Ye, Q. et al. TRIP13 is a protein-remodeling AAA+ ATPase that catalyzes MAD2 conformation switching. eLife 4, e07367 (2015).
Habu, T., Kim, S. H., Weinstein, J. & Matsumoto, T. Identification of a MAD2-binding protein, CMT2, and its role in mitosis. EMBO J. 21, 6419–6428 (2002).
Eytan, E. et al. Disassembly of mitotic checkpoint complexes by the joint action of the AAA-ATPase TRIP13 and p31comet. Proc. Natl Acad. Sci. USA 111, 12019–12024 (2014).
Ma, H. T. & Poon, R. Y. C. TRIP13 regulates both the activation and inactivation of the spindle-assembly checkpoint. Cell Rep. 14, 1086–1099 (2016).
Ma, H. T. & Poon, R. Y. C. TRIP13 functions in the establishment of the spindle assembly checkpoint by replenishing O-MAD2. Cell Rep. 22, 1439–1450 (2018).
Brulotte, M. L. et al. Mechanistic insight into TRIP13-catalyzed Mad2 structural transition and spindle checkpoint silencing. Nat. Commun. 8, 1956 (2017).
Kim, D. H. et al. TRIP13 and APC15 drive mitotic exit by turnover of interphase- and unattached kinetochore-produced MCC. Nat. Commun. 9, 4354 (2018).
Westhorpe, F. G., Tighe, A., Lara-Gonzalez, P. & Taylor, S. S. p31comet-mediated extraction of Mad2 from the MCC promotes efficient mitotic exit. J. Cell Sci. 124, 3905–3916 (2011).
Xia, G. et al. Conformation-specific binding of p31comet antagonizes the function of Mad2 in the spindle checkpoint. EMBO J. 23, 3133–3143 (2004).
Mapelli, M. et al. Determinants of conformational dimerization of Mad2 and its inhibition by p31comet. EMBO J. 25, 1273–1284 (2006).
Yang, M. et al. p31comet blocks Mad2 activation through structural mimicry. Cell 131, 744–755 (2007).
Wang, K. et al. Thyroid hormone receptor interacting protein 13 (TRIP13) AAA-ATPase is a novel mitotic checkpoint-silencing protein. J. Biol. Chem. 289, 23928–23937 (2014).
Alfieri, C., Chang, L. & Barford, D. Mechanism for remodelling of the cell cycle checkpoint protein MAD2 by the ATPase TRIP13. Nature 559, 274–278 (2018).
Kulukian, A., Han, J. S. & Cleveland, D. W. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev. Cell 16, 105–117 (2009).
Nelson, C. R., Hwang, T., Chen, P.-H. & Bhalla, N. TRIP13PCH-2 promotes Mad2 localization to unattached kinetochores in the spindle checkpoint response. J. Cell Biol. 211, 503–516 (2015).
Kaisari, S. et al. Role of Polo-like kinase 1 in the regulation of the action of p31comet in the disassembly of mitotic checkpoint complexes. Proc. Natl Acad. Sci. USA 116, 11725–11730 (2019).
Date, D. A., Burrows, A. C. & Summers, M. K. Phosphorylation regulates the p31Comet-mitotic arrest-deficient 2 (Mad2) interaction to promote spindle assembly checkpoint (SAC) activity. J. Biol. Chem. 289, 11367–11373 (2014).
Ciliberto, A. & Shah, J. V. A quantitative systems view of the spindle assembly checkpoint. EMBO J. 28, 2162–2173 (2009).
Aravamudhan, P., Goldfarb, A. A. & Joglekar, A. P. The kinetochore encodes a mechanical switch to disrupt spindle assembly checkpoint signalling. Nat. Cell Biol. 17, 868–879 (2015). This study based on budding yeast illustrates how positioning of Mps1 activity relative to its key SAC substrate Knl1 (Spc105 in yeast) can activate or silence the SAC.
Hayward, D. et al. Checkpoint signaling and error correction require regulation of the MPS1 T-loop by PP2A-B56. J. Cell Biol. 218, 3188–3199 (2019).
Pinsky, B. A., Nelson, C. R. & Biggins, S. Protein phosphatase 1 regulates exit from the spindle checkpoint in budding yeast. Curr. Biol. 19, 1182–1187 (2009).
Vanoosthuyse, V. & Hardwick, K. G. A novel protein phosphatase 1-dependent spindle checkpoint silencing mechanism. Curr. Biol. 19, 1–6 (2009).
Espert, A. et al. PP2A-B56 opposes Mps1 phosphorylation of Knl1 and thereby promotes spindle assembly checkpoint silencing. J. Cell Biol. 206, 833–842 (2014).
Nijenhuis, W., Vallardi, G., Teixeira, A., Kops, G. J. P. L. & Saurin, A. T. Negative feedback at kinetochores underlies a responsive spindle checkpoint signal. Nat. Cell Biol. 16, 1257–1264 (2014). This paper illustrates a phosphatase feedback at kinetochores that primes the SAC for rapid activation upon detachment.
Rosenberg, J. S., Cross, F. R. & Funabiki, H. KNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr. Biol. 21, 942–947 (2011).
Moura, M. et al. Protein phosphatase 1 inactivates Mps1 to ensure efficient spindle assembly checkpoint silencing. eLife 6, e25366 (2017).
Cordeiro, M. H., Smith, R. J. & Saurin, A. T. Kinetochore phosphatases suppress autonomous Polo-like kinase 1 activity to control the mitotic checkpoint. J. Cell Biol. 219, e202002020 (2020).
von Schubert, C. et al. Plk1 and Mps1 cooperatively regulate the spindle assembly checkpoint in human cells. Cell Rep. 12, 66–78 (2015).
Ikeda, M. & Tanaka, K. Plk1 bound to Bub1 contributes to spindle assembly checkpoint activity during mitosis. Sci. Rep. 7, 8794 (2017).
Espeut, J. et al. Natural loss of Mps1 kinase in nematodes uncovers a role for polo-like kinase 1 in spindle checkpoint initiation. Cell Rep. 12, 58–65 (2015).
Liu, D. et al. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J. Cell Biol. 188, 809–820 (2010).
Gelens, L., Qian, J., Bollen, M. & Saurin, A. T. The importance of kinase-phosphatase integration: lessons from mitosis. Trends Cell Biol. 28, 6–21 (2018).
Howell, B. J. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 155, 1159–1172 (2001).
Griffis, E. R., Stuurman, N. & Vale, R. D. Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J. Cell Biol. 177, 1005–1015 (2007).
Wojcik, E. et al. Kinetochore dynein: Its dynamics and role in the transport of the Rough deal checkpoint protein. Nat. Cell Biol. 3, 1001–1007 (2001).
Kops, G. J. P. L. & Shah, J. V. Connecting up and clearing out: how kinetochore attachment silences the spindle assembly checkpoint. Chromosoma 121, 509–525 (2012).
O’Connell, C. B. et al. The spindle assembly checkpoint is satisfied in the absence of interkinetochore tension during mitosis with unreplicated genomes. J. Cell Biol. 183, 29–36 (2008).
Drpic, D., Pereira, A. J., Barisic, M., Maresca, T. J. & Maiato, H. Polar ejection forces promote the conversion from lateral to end-on kinetochore-microtubule attachments on mono-oriented chromosomes. Cell Rep. 13, 460–468 (2015).
Dudka, D. et al. Complete microtubule-kinetochore occupancy favours the segregation of merotelic attachments. Nat. Commun. 9, 2042 (2018).
Etemad, B., Kuijt, T. E. F. & Kops, G. J. P. L. Kinetochore–microtubule attachment is sufficient to satisfy the human spindle assembly checkpoint. Nat. Commun. 6, 8987 (2015).
Tauchman, E. C., Boehm, F. J. & DeLuca, J. G. Stable kinetochore–microtubule attachment is sufficient to silence the spindle assembly checkpoint in human cells. Nat. Commun. 6, 10036 (2015).
Kuhn, J. & Dumont, S. Spindle assembly checkpoint satisfaction occurs via end-on but not lateral attachments under tension. J. Cell Biol. 216, 1533–1542 (2017).
Magidson, V. et al. Unattached kinetochores rather than intrakinetochore tension arrest mitosis in Taxol-treated cells. J. Cell Biol. 212, 307–319 (2016). Together with Etemad et al. (2015), Tauchman et al. (2015) and Kuhn and Dumont (2017), this work shows that in human cells the SAC is silenced by microtubule attachment to kinetochores rather than tension between or within kinetochores.
Roscioli, E. et al. Ensemble-level organization of human kinetochores and evidence for distinct tension and attachment sensors. Cell Rep. 31, 107535 (2020). This study reports on distinct rearrangements within the ensemble-level 3D organization of human kinetochores upon the loss of attachment or tension.
Burroughs, N. J., Harry, E. F. & McAinsh, A. D. Super-resolution kinetochore tracking reveals the mechanisms of human sister kinetochore directional switching. eLife 4, e09500 (2015).
Wan, X., Cimini, D., Cameron, L. A. & Salmon, E. D. The coupling between sister kinetochore directional instability and oscillations in centromere stretch in metaphase PtK1 cells. Mol. Biol. Cell 23, 1035–1046 (2012).
Hayward, D., Roberts, E. & Gruneberg, U. MPS1 localizes to end-on microtubule-attached kinetochores to promote microtubule release. Curr. Biol. 32, 5200–5208.e8 (2022).
Howell, B. J. et al. Spindle checkpoint protein dynamics at kinetochores in living cells. Curr. Biol. 14, 953–964 (2004).
Wan, X. et al. Protein architecture of the human kinetochore microtubule attachment site. Cell 137, 672–684 (2009).
Uchida, K. S. K. et al. Kinetochore stretching inactivates the spindle assembly checkpoint. J. Cell Biol. 184, 383–390 (2009).
Maresca, T. J. & Salmon, E. D. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J. Cell Biol. 184, 373–381 (2009).
Uchida, K. S. K. et al. Kinetochore stretching-mediated rapid silencing of the spindle-assembly checkpoint required for failsafe chromo. Curr. Biol. 31, 1581–1591 (2021).
Conway, W. et al. Self-organization of kinetochore-fibers in human mitotic spindles. eLife 11, e75458 (2022).
Drpic, D. et al. Chromosome segregation is biased by kinetochore size. Curr. Biol. 28, 1344–1356.e5 (2018).
Comings, D. E. & Okada, T. A. Fine structure of kinetochore in Indian muntjac. Exp. Cell Res. 67, 97–110 (1971).
Senaratne, A. P., Cortes-Silva, N. & Drinnenberg, I. A. Evolution of holocentric chromosomes: drivers, diversity, and deterrents. Semin. Cell Dev. Biol. 127, 90–99 (2022).
Kukreja, A. A., Kavuri, S. & Joglekar, A. P. Microtubule attachment and centromeric tension shape the protein architecture of the human kinetochore. Curr. Biol. 30, 4869–4881.e5 (2020).
Etemad, B. et al. Spindle checkpoint silencing at kinetochores with submaximal microtubule occupancy. J. Cell Sci. 132, jcs231589 (2019).
Kuhn, J. & Dumont, S. Mammalian kinetochores count attached microtubules in a sensitive and switch-like manner. J. Cell Biol. 218, 3583–3596 (2019). Together with Dudka et al. (2018) and Etemad et al. (2019), this work shows that SAC signalling can fully switch off at human kinetochores that have not yet bound a full complement of microtubules.
Zaytsev, A. V., Sundin, L. J. R., DeLuca, K. F., Grishchuk, E. L. & DeLuca, J. G. Accurate phosphoregulation of kinetochore–microtubule affinity requires unconstrained molecular interactions. J. Cell Biol. 206, 45–59 (2014).
Joglekar, A. P. et al. Molecular architecture of the kinetochore–microtubule attachment site is conserved between point and regional centromeres. J. Cell Biol. 181, 587–594 (2008).
Joglekar, A. P., Bouck, D. C., Molk, J. N., Bloom, K. S. & Salmon, E. D. Molecular architecture of a kinetochore–microtubule attachment site. Nat. Cell Biol. 8, 581–585 (2006).
Zhao, W. & Jensen, G. J. In situ architecture of human kinetochore-microtubule interface visualized by cryo-electron tomography. Preprint at bioRxiv https://doi.org/10.1101/2022.02.17.480955 (2022).
Etemad, B. & Kops, G. J. Attachment issues: kinetochore transformations and spindle checkpoint silencing. Curr. Opin. Cell Biol. 39, 101–108 (2016).
Yoo, T. Y. et al. Measuring NDC80 binding reveals the molecular basis of tension-dependent kinetochore-microtubule attachments. eLife 7, e36392 (2018).
Renda, F. et al. Effects of malleable kinetochore morphology on measurements of intrakinetochore tension. Open Biol. 10, 200101 (2020).
Sacristan, C. et al. Condensin reorganizes centromeric chromatin during mitotic entry into a bipartite structure stabilized by cohesin. Preprint at bioRxiv https://doi.org/10.1101/2022.08.01.502248 (2022).
Rieder, C. L., Schultz, A., Cole, R. & Sluder, G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 127, 1301–1310 (1994).
Vleugel, M. et al. Sequential multisite phospho-regulation of KNL1–BUB3 interfaces at mitotic kinetochores. Mol. Cell 57, 824–835 (2015).
Chen, C. et al. Ectopic activation of the spindle assembly checkpoint signaling cascade reveals its biochemical design. Curr. Biol. 29, 104–119.e10 (2019). This study shows signalling principles of an elegant ectopic SAC system to suggest that the signal output per kinetochore and the number of signalling kinetochores has an inverse, non-linear relationship, enabling the SAC to approximate switch-like behaviour.
Vleugel, M. et al. Arrayed BUB recruitment modules in the kinetochore scaffold KNL1 promote accurate chromosome segregation. J. Cell Biol. 203, 943–955 (2013).
Zhang, G., Lischetti, T. & Nilsson, J. A minimal number of MELT repeats supports all the functions of KNL1 in chromosome segregation. J. Cell Sci. 127, 871–884 (2014).
Suijkerbuijk, S. J. E., Vleugel, M., Teixeira, A. & Kops, G. J. P. L. Integration of kinase and phosphatase activities by BUBR1 ensures formation of stable kinetochore-microtubule attachments. Dev. Cell 23, 745–755 (2012).
Kruse, T. et al. Direct binding between BubR1 and B56-PP2A phosphatase complexes regulate mitotic progression. J. Cell Sci. 126, 1086–1092 (2013).
Roy, B., Han, S. J. Y., Fontan, A. N. & Joglekar, A. P. The copy-number and varied strengths of melt motifs in spc105 balance the strength and responsiveness of the spindle assembly checkpoint. eLife 9, e55096 (2020).
Corno, A. et al. A bifunctional kinase-phosphatase module integrates mitotic checkpoint and error-correction signalling to ensure mitotic fidelity. Preprint at bioRxiv https://doi.org/10.1101/2022.05.22.492960 (2022).
Heinrich, S. et al. Determinants of robustness in spindle assembly checkpoint signalling. Nat. Cell Biol. 15, 1328–1339 (2013).
Galli, M. & Morgan, D. O. Cell size determines the strength of the spindle assembly checkpoint during embryonic development. Dev. Cell 36, 344–352 (2016).
Li, R. & Murray, A. W. Feedback control of mitosis in budding yeast. Cell 66, 519–531 (1991).
Carmena, M., Wheelock, M., Funabiki, H. & Earnshaw, W. C. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 13, 789–803 (2012).
Ciferri, C. et al. Implications for kinetochore–microtubule attachment from the structure of an engineered Ndc80 complex. Cell 133, 427–439 (2008).
Cheeseman, I. M., Chappie, J. S., Wilson-Kubalek, E. M. & Desai, A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127, 983–997 (2006).
Ditchfield, C. et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161, 267–280 (2003).
Hauf, S. et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore–microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161, 281–294 (2003).
Saurin, A. T., van der Waal, M. S., Medema, R. H., Lens, S. M. A. & Kops, G. J. P. L. Aurora B potentiates Mps1 activation to ensure rapid checkpoint establishment at the onset of mitosis. Nat. Commun. 2, 316 (2011).
Pachis, S. T., Hiruma, Y., Perrakis, A. & Kops, G. J. P. L. Interactions between N-terminal modules in MPS1 enable spindle checkpoint silencing. Cell Rep. 26, 2101–2112 (2018).
Nijenhuis, W. et al. A TPR domain-containing N-terminal module of MPS1 is required for its kinetochore localization by Aurora B. J. Cell Biol. 201, 217–231 (2013).
Santaguida, S., Vernieri, C., Villa, F., Ciliberto, A. & Musacchio, A. Evidence that Aurora B is implicated in spindle checkpoint signalling independently of error correction. EMBO J. 30, 1508–1519 (2011).
Roy, B., Han, S. J. Y., Fontan, A. N., Jema, S. & Joglekar, A. P. Aurora B phosphorylates Bub1 to promote spindle assembly checkpoint signaling. Curr. Biol. 32, 237–247 (2021).
Hindriksen, S., Lens, S. M. A. & Hadders, M. A. The ins and outs of Aurora B inner centromere localization. Front. Cell Dev. Biol. 5, 112 (2017).
Hengeveld, R. C. C., Vromans, M. J. M., Vleugel, M., Hadders, M. A. & Lens, S. M. A. Inner centromere localization of the CPC maintains centromere cohesion and allows mitotic checkpoint silencing. Nat. Commun. 8, 15542 (2017).
Broad, A. J., DeLuca, K. F. & DeLuca, J. G. Aurora B kinase is recruited to multiple discrete kinetochore and centromere regions in human cells. J. Cell Biol. 219, e201905144 (2020).
Acknowledgements
The authors thank S. Biggins and J. Millar for comments on the manuscript, C. Conway for helping devise Fig. 7a and the three reviewers for their thorough and constructive feedback. A.D.M. is supported by a Wellcome collaborator award (215625) and the Biotechnology and Biological Sciences Research Council (BB/R009503/1).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks David Barford, who co-reviewed with Elyse Fischer, Ajit Joglekar, who co-reviewed with Dana Beseiso, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- AAA+ ATPase
-
A class of protein enzymes that use ATP hydrolysis for remodelling or translocating macromolecules.
- Aneuploidy
-
The state where a cell of a given species has numerous chromosomes that deviates from a multiple of the haploid set of that species.
- Centromeric chromatin
-
Specialized chromatin at centromeres that define the site of kinetochore assembly, in which a subset of nucleosomes contains the histone H3 variant centromere protein A (CENP-A).
- Chromosome bi-orientation
-
The configuration of a duplicated chromosome on a bipolar spindle in mitosis in which the kinetochore of one sister chromatid is attached to one pole whereas the kinetochore of the other sister chromatid is attached to the other pole.
- Chromosomal passenger complex
-
(CPC). A tetrameric protein complex containing the Aurora B kinase that locates to and functions at various locations in mitotic cells, regulating sister chromatid cohesion, attachment error correction, spindle checkpoint signalling and cytokinesis.
- Degron
-
A short linear amino acid sequence motif that is recognized by the cellular protein degradation machinery for targeting the host protein for degradation.
- End-on interactions
-
Interactions of kinetochore proteins with microtubules near their plus ends, resulting in plus-end embedding into kinetochores and subsequent ability of plus-end depolymerization to exert pulling force on the chromosome.
- Holocentric chromosomes
-
Chromosomes, natural to certain species, that contain microtubule-binding kinetochores along their entire length rather than at a single defined location (that is, the centromere).
- Interphase
-
The phase between two mitosis events, encompassing the cell cycle phases G0, G1, S and G2.
- Kinetochore fibres
-
Bundles of microtubules that together connect to a single kinetochore.
- NDC80 complex
-
A tetrameric protein complex assembled from SPC24, SPC25, NUF2 and NDC80 (also known as HEC1), of which the latter two components directly bind to microtubules.
- Nuclear envelope breakdown
-
The process of disassembling the nuclear envelope at the start of mitosis in order to enable access of chromosomes to cytoplasmic microtubules.
- Paralogues
-
Pairs of homologous genes that have diverged in sequence following gene duplication during evolution.
- Spindle poles
-
The regions of the spindle where the minus ends of microtubules come together. In human somatic cells, the centrosomes form the two spindle poles.
- Taxol
-
Brand name of paclitaxel, a cytostatic anticancer drug that inhibits microtubule depolymerization, originally made from extract of the Taxus brevifolia tree.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McAinsh, A.D., Kops, G.J.P.L. Principles and dynamics of spindle assembly checkpoint signalling. Nat Rev Mol Cell Biol 24, 543–559 (2023). https://doi.org/10.1038/s41580-023-00593-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-023-00593-z
This article is cited by
-
The yin and yang of chromosomal instability in prostate cancer
Nature Reviews Urology (2024)
-
Calreticulin and JAK2V617F driver mutations induce distinct mitotic defects in myeloproliferative neoplasms
Scientific Reports (2024)
-
Expression of the checkpoint kinase BUB1 is a predictor of response to cancer therapies
Scientific Reports (2024)
-
Structure of the human outer kinetochore KMN network complex
Nature Structural & Molecular Biology (2024)
-
The two sides of chromosomal instability: drivers and brakes in cancer
Signal Transduction and Targeted Therapy (2024)