Key Points
-
Developmental phase switches and identity changes need global changes in gene expression patterns.
-
Genome-wide chromatin immunoprecipitation approaches have provided insights into the transcriptional networks and topology of the networks that underlie phase changes and identity specification.
-
Phase switches involve suppression of the preceding developmental programme by negative feedback regulation and activation of the next developmental programme by positive (autoregulatory) regulation mechanisms.
-
Combinatorial interaction among transcription factors determines regulatory specificity and defines the set of target genes.
-
Key regulators are transcription factors and chromatin modifiers
-
These key regulators form protein complexes and the transcription factors may recruit the chromatin modifiers to specific loci.
Abstract
Unlike animals, plants produce new organs throughout their life cycle using pools of stem cells that are organized in meristems. Although many key regulators of meristem and organ identities have been identified, it is still not well understood how they function at the molecular level and how they can switch an entire developmental programme in which thousands of genes are involved. Recent advances in the genome-wide identification of target genes controlled by key plant transcriptional regulators and their interactions with epigenetic factors provide new insights into general transcriptional regulatory mechanisms that control switches of developmental programmes and cell fates in complex organisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Walbot, V. & Evans, M. M. Unique features of the plant life cycle and their consequences. Nature Rev. Genet. 4, 369–379 (2003).
Busch, W. & Benfey, P. N. Information processing without brains — the power of intercellular regulators in plants. Development 137, 1215–1226 (2010).
Graf, T. & Enver, T. Forcing cells to change lineages. Nature 462, 587–594 (2009).
Scheres, B. Stem-cell niches: nursery rhymes across kingdoms. Nature Rev. Mol. Cell Biol. 8, 345–354 (2007).
Letsou, A. & Bohmann, D. Small flies — big discoveries: nearly a century of Drosophila genetics and development. Dev. Dyn. 232, 526–528 (2005).
Ho, L. & Crabtree, G. R. Chromatin remodelling during development. Nature 463, 474–484 (2010).
Desvoyes, B., Sanchez, M. P., Ramirez-Parra, E. & Gutierrez, C. Impact of nucleosome dynamics and histone modifications on cell proliferation during Arabidopsis development. Heredity 105, 80–91 (2010).
Causier, B., Schwarz-Sommer, Z. & Davies, B. Floral organ identity: 20 years of ABCs. Semin. Cell Dev. Biol. 21, 73–79 (2010).
Honma, T. & Goto, K. Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409, 525–529 (2001).
Pelaz, S., Tapia-Lopez, R., Alvarez-Buylla, E. R. & Yanofsky, M. F. Conversion of leaves into petals in Arabidopsis. Curr. Biol. 11, 182–184 (2001).
Theissen, G. Development of floral organ identity: stories from the MADS house. Curr. Opin. Plant Biol. 4, 75–85 (2001).
Ito, T. et al. The homeotic protein AGAMOUS controls microsporogenesis by regulation of SPOROCYTELESS. Nature 430, 356–360 (2004).
Gomez-Mena, C., de Folter, S., Costa, M. M. R., Angenent, G. C. & Sablowski, R. Transcriptional program controlled by the floral homeotic gene AGAMOUS during early organogenesis. Development 132, 429–438 (2005).
Wellmer, F., Alves-Ferreira, M., Dubois, A., Riechmann, J. L. & Meyerowitz, E. M. Genome-wide analysis of gene expression during early Arabidopsis flower development. PLOS Genet. 2, 1012–1024 (2006).
Morohashi, K. & Grotewold, E. A systems approach reveals regulatory circuitry for Arabidopsis trichome initiation by the GL3 and GL1 selectors. PLoS Genet. 5, e1000396 (2009).
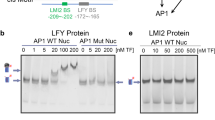
Kaufmann, K. et al. Orchestration of floral initiation by APETALA1. Science 328, 85–89 (2010). This publication describes the early set of target genes of the MADS-domain transcription factor AP1 and the dynamics of regulation. It demonstrates the two main roles of a transcription factor that controls a switch of meristem identity: activation of a developmental programme and repression of previous or inappropriate programmes.
Farnham, P. J. Insights from genomic profiling of transcription factors. Nature Rev. Genet. 10, 605–616 (2009).
Herskowitz, I. Master regulatory loci in yeast and lambda. Cold Spring Harb. Symp. Quant. Biol. 50, 565–574 (1985).
Lewis, E. B. Clusters of master control genes regulate the development of higher organisms. JAMA 267, 1524–1531 (1992).
Meyerowitz, E. M. Plants compared to animals: the broadest comparative study of development. Science 295, 1482–1485 (2002).
Gehring, W. J. & Ikeo, K. Pax 6 — mastering eye morphogenesis and eye evolution. Trends Genet. 15, 371–377 (1999).
Yu, H. Y. & Gerstein, M. Genomic analysis of the hierarchical structure of regulatory networks. Proc. Natl Acad. Sci. USA 103, 14724–14731 (2006).
Akam, M. Hox genes: from master genes to micromanagers. Curr. Biol. 8, R676–R678 (1998).
Babu, M. M., Luscombe, N. M., Aravind, L., Gerstein, M. & Teichmann, S. A. Structure and evolution of transcriptional regulatory networks. Curr. Opin. Struct. Biol. 14, 283–291 (2004).
Jothi, R. et al. Genomic analysis reveals a tight link between transcription factor dynamics and regulatory network architecture. Mol. Syst. Biol. 5, e294 (2009).
Alon, U. Network motifs: theory and experimental approaches. Nature Rev. Genet. 8, 450–461 (2007).
Bar-Yam, Y., Harmon, D. & de Bivort, B. Systems biology: attractors and democratic dynamics. Science 323, 1016–1017 (2009).
Busch, W. et al. Transcriptional control of a plant stem cell niche. Dev. Cell 18, 849–861 (2010). This paper describes direct downstream target genes of the plant stem cell regulator WUSCHEL. It substantiates the complex interplay between intercellular signalling and transcriptional feedback in the plant stem cell niche.
Kempin, S. A., Savidge, B. & Yanofsky, M. F. Molecular basis of the cauliflower phenotype in Arabidopsis. Science 267, 522–525 (1995).
Liu, C. et al. Specification of Arabidopsis floral meristem identity by repression of flowering time genes. Development 134, 1901–1910 (2007).
Wigge, P. A. et al. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309, 1056–1059 (2005).
Wang, J. W., Czech, B. & Weigel, D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138, 738–749 (2009).
Weigel, D., Alvarez, J., Smyth, D. R., Yanofsky, M. F. & Meyerowitz, E. M. LEAFY controls floral meristem identity in Arabidopsis. Cell 69, 843–859 (1992).
Parcy, F., Nilsson, O., Busch, M. A., Lee, I. & Weigel, D. A genetic framework for floral patterning. Nature 395, 561–566 (1998).
Liljegren, S. J., Gustafson-Brown, C., Pinyopich, A., Ditta, G. S. & Yanofsky, M. F. Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell 11, 1007–1018 (1999).
Immink, R. G. et al. SEPALLATA3: the 'glue' for MADS box transcription factor complex formation. Genome Biol. 10, R24 (2009).
Pelaz, S., Ditta, G. S., Baumann, E., Wisman, E. & Yanofsky, M. F. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405, 200–203 (2000).
Kaufmann, K. et al. Target genes of the MADS transcription factor SEPALLATA3: integration of developmental and hormonal pathways in the Arabidopsis flower. PLoS Biol. 7, e1000090 (2009).
Ito, T., Ng, K. H., Lim, T. S., Yu, H. & Meyerowitz, E. M. The homeotic protein AGAMOUS controls late stamen development by regulating a jasmonate biosynthetic gene in Arabidopsis. Plant Cell 19, 3516–3529 (2007).
Drews, G. N., Bowman, J. L. & Meyerowitz, E. M. Negative regulation of the Arabidopsis homeotic gene AGAMOUS by the APETALA2 product. Cell 65, 991–1002 (1991).
Wurschum, T., Gross-Hardt, R. & Laux, T. APETALA2 regulates the stem cell niche in the Arabidopsis shoot meristem. Plant Cell 18, 295–307 (2006).
Ohto, M. A., Floyd, S. K., Fischer, R. L., Goldberg, R. B. & Harada, J. J. Effects of APETALA2 on embryo, endosperm, and seed coat development determine seed size in Arabidopsis. Sex. Plant Reprod. 22, 277–289 (2009).
Yant, L. et al. Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 22, 2156–2170 (2010).
Aukerman, M. J. & Sakai, H. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15, 2730–2741 (2003).
Schmid, M. et al. Dissection of floral induction pathways using global expression analysis. Development 130, 6001–6012 (2003).
Mathieu, J., Yant, L. J., Murdter, F., Kuttner, F. & Schmid, M. Repression of flowering by the miR172 target, SMZ. PLoS Biol. 7, e1000148 (2009).
Ha, C. M., Jun, J. H., Nam, H. G. & Fletcher, J. C. BLADE-ON-PETIOLE 1 and 2 control Arabidopsis lateral organ fate through regulation of LOB domain and adaxial–abaxial polarity genes. Plant Cell 19, 1809–1825 (2007).
Rast, M. I. & Simon, R. The meristem-to-organ boundary: more than an extremity of anything. Curr. Opin. Genet. Dev. 18, 287–294 (2008).
Gutierrez, C. Coupling cell proliferation and development in plants. Nature Cell Biol. 7, 535–541 (2005).
Xie, Z. et al. Regulation of cell proliferation in the stomatal lineage by the Arabidopsis MYB FOUR LIPS via direct targeting of core cell cycle genes. Plant Cell 22, 2306–2321 (2010).
Sozzani, R. et al. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature 466, 128–132 (2010). This is one of several publications that describe direct regulation of core cell cycle genes by regulators of cell identity and patterning in plants. The authors combined ChIP–chip with genome-wide approaches to measure cell-type specific expression dynamics.
Lee, J., Oh, M., Park, H. & Lee, I. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates LEAFY. Plant J. 55, 832–843 (2008).
Gregis, V., Sessa, A., Colombo, L. & Kater, M. M. AGL24, SHORT VEGETATIVE PHASE, and APETALA1 redundantly control AGAMOUS during early stages of flower development in Arabidopsis. Plant Cell 18, 1373–1382 (2006).
Cole, M., Nolte, C. & Werr, W. Nuclear import of the transcription factor SHOOT MERISTEMLESS depends on heterodimerization with BLH proteins expressed in discrete sub-domains of the shoot apical meristem of Arabidopsis thaliana. Nucleic Acids Res. 34, 1281–1292 (2006).
Rutjens, B. et al. Shoot apical meristem function in Arabidopsis requires the combined activities of three BEL1-like homeodomain proteins. Plant J. 58, 641–654 (2009).
Chandler, J. W., Cole, M., Flier, A., Grewe, B. & Werr, W. The AP2 transcription factors DORNROSCHEN and DORNROSCHEN-LIKE redundantly control Arabidopsis embryo patterning via interaction with PHAVOLUTA. Development 134, 1653–1662 (2007).
Cui, H. C. et al. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science 316, 421–425 (2007).
Zhao, M., Morohashi, K., Hatlestad, G., Grotewold, E. & Lloyd, A. The TTG1–bHLH–MYB complex controls trichome cell fate and patterning through direct targeting of regulatory loci. Development 135, 1991–1999 (2008).
Chandler, J. W., Cole, M., Flier, A. & Werr, W. BIM1, a bHLH protein involved in brassinosteroid signalling, controls Arabidopsis embryonic patterning via interaction with DORNROSCHEN and DORNROSCHEN-LIKE. Plant Mol. Biol. 69, 57–68 (2009).
Guo, M. J., Thomas, J., Collins, G. & Timmermans, M. C. P. Direct repression of KNOX loci by the ASYMMETRIC LEAVES1 complex of Arabidopsis. Plant Cell 20, 48–58 (2008).
Smith, H. M. S. & Hake, S. The interaction of two homeobox genes, BREVIPEDICELLUS and PENNYWISE, regulates internode patterning in the Arabidopsis inflorescence. Plant Cell 15, 1717–1727 (2003).
Kanrar, S., Onguka, O. & Smith, H. M. S. Arabidopsis inflorescence architecture requires the activities of KNOX–BELL homeodomain heterodimers. Planta 224, 1163–1173 (2006).
Gregis, V., Sessa, A., Colombo, L. & Kater, M. M. AGAMOUS-LIKE24 and SHORT VEGETATIVE PHASE determine floral meristem identity in Arabidopsis. Plant J. 56, 891–902 (2008).
Wenkel, S., Emery, J., Hou, B. H., Evans, M. M. S. & Barton, M. K. A feedback regulatory module formed by LITTLE ZIPPER and HD-ZIPIII genes. Plant Cell 19, 3379–3390 (2007).
Zinzen, R. P., Girardot, C., Gagneur, J., Braun, M. & Furlong, E. E. Combinatorial binding predicts spatio-temporal cis-regulatory activity. Nature 462, 65–70 (2009).
Siegal-Gaskins, D., Grotewold, E. & Smith, G. D. The capacity for multistability in small gene regulatory networks. BMC Syst. Biol. 3, 96 (2009).
Kitano, H. Biological robustness. Nature Rev. Genet. 5, 826–837 (2004).
Lenser, T., Theissen, G. & Dittrich, P. Developmental robustness by obligate interaction of class B floral homeotic genes and proteins. PLoS Comput. Biol. 5, e1000264 (2009).
Smith, Z. R. & Long, J. A. Control of Arabidopsis apical–basal embryo polarity by antagonistic transcription factors. Nature 464, U423–U121 (2010). This paper describes the antagonistic action of transcription factors that specify the root and shoot pole in the Arabidopsis embryo. It also shows that the transcriptional co-repressor TOPLESS controls embryo polarity by direct repression of PLETHORA genes, which are master regulators of root fate.
Ha, C. M. et al. The BLADE-ON-PETIOLE 1 gene controls leaf pattern formation through the modulation of meristematic activity in Arabidopsis. Development 130, 161–172 (2003).
Jun, J. H., Ha, C. M. & Fletcher, J. C. BLADE-ON-PETIOLE1 coordinates organ determinacy and axial polarity in Arabidopsis by directly activating ASYMMETRIC LEAVES 2. Plant Cell 22, 62–76 (2010).
Wu, G. et al. KANADI1 regulates adaxial–abaxial polarity in Arabidopsis by directly repressing the transcription of ASYMMETRIC LEAVES 2. Proc. Natl Acad. Sci. USA 105, 16392–16397 (2008).
Schwarz-Sommer, Z. et al. Characterization of the Antirrhinum floral homeotic MADS--box gene deficiens – evidence for DNA binding and autoregulation of its persistent expression throughout flower development. EMBO J. 11, 251–263 (1992).
Michaels, S. D. et al. AGL24 acts as a promoter of flowering in Arabidopsis and is positively regulated by vernalization. Plant J. 33, 867–874 (2003).
Liu, C. et al. Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development 135, 1481–1491 (2008).
Melzer, S. et al. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nature Genet. 40, 1489–1492 (2008).
Okamuro, J. K., denBoer, B. G. W., LotysPrass, C., Szeto, W. & Jofuku, K. D. Flowers into shoots: photo and hormonal control of a meristem identity switch in Arabidopsis. Proc. Natl Acad. Sci. USA 93, 13831–13836 (1996).
Yadav, R. K., Tavakkoli, M. & Reddy, G. V. WUSCHEL mediates stem cell homeostasis by regulating stem cell number and patterns of cell division and differentiation of stem cell progenitors. Development 137, 3581–3589 (2010).
Flaus, A., Martin, D. M. A., Barton, G. J. & Owen-Hughes, T. Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res. 34, 2887–2905 (2006).
Hennig, L. & Derkacheva, M. Diversity of Polycomb group complexes in plants: same rules, different players? Trends Genet. 25, 414–423 (2009).
Chen, L. J., Diao, Z. Y., Specht, C. & Sung, Z. R. Molecular evolution of VEF-domain-containing PcG genes in plants. Mol. Plant 2, 738–754 (2009).
Exner, V. & Hennig, L. Chromatin rearrangements in development. Curr. Opin. Plant Biol. 11, 64–69 (2008).
Kaya, H. et al. FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems. Cell 104, 131–142 (2001).
Exner, V., Taranto, P., Schonrock, N., Gruissem, W. & Hennig, L. Chromatin assembly factor CAF-1 is required for cellular differentiation during plant development. Development 133, 4163–4172 (2006).
Ono, T. et al. Chromatin assembly factor 1 ensures the stable maintenance of silent chromatin states in Arabidopsis. Genes Cells 11, 153–162 (2006).
Farrona, S., Hurtado, L., Bowman, J. L. & Reyes, J. C. The Arabidopsis thaliana SNF2 homolog AtBRM controls shoot development and flowering. Development 131, 4965–4975 (2004).
Hurtado, L., Farrona, S. & Reyes, J. C. The putative SWI/SNF complex subunit BRAHMA activates flower homeotic genes in Arabidopsis thaliana. Plant Mol. Biol. 62, 291–304 (2006).
Bezhani, S. et al. Unique, shared, and redundant roles for the Arabidopsis SWI/SNF chromatin remodeling ATPases BRAHMA and SPLAYED. Plant Cell 19, 403–416 (2007).
Tang, X. R. et al. The Arabidopsis BRAHMA chromatin-remodeling ATPase is involved in repression of seed maturation genes in leaves. Plant Physiol. 147, 1143–1157 (2008).
Wagner, D. & Meyerowitz, E. M. SPLAYED, a novel SWI/SNF ATPase homolog, controls reproductive development in Arabidopsis. Curr. Biol. 12, 85–94 (2002).
Kwon, C. S., Chen, C. B. & Wagner, D. WUSCHEL is a primary target for transcriptional regulation by SPLAYED in dynamic control of stem cell fate in Arabidopsis. Genes Dev. 19, 992–1003 (2005).
Kumar, S. V. & Wigge, P. A. H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140, 136–147 (2010).
Costa, S. & Shaw, P. Chromatin organization and cell fate switch respond to positional information in Arabidopsis. Nature 439, 493–496 (2006). Using microscopic techniques, the authors show local changes in chromatin organization linked with the activation of GLABRA2 , a gene that controls epidermal cell differentiation.
Caro, E., Castellano, M. M. & Gutierrez, C. A chromatin link that couples cell division to root epidermis patterning in Arabidopsis. Nature 447, 213–217 (2007).
Servet, C., Conde, E. S. N. & Zhou, D. X. Histone acetyltransferase AtGCN5/HAG1 is a versatile regulator of developmental and inducible gene expression in Arabidopsis. Mol. Plant 3, 670–677 (2010).
Kornet, N. & Scheres, B. Members of the GCN5 histone acetyltransferase complex regulate PLETHORA-mediated root stem cell niche maintenance and transit amplifying cell proliferation in Arabidopsis. Plant Cell 21, 1070–1079 (2009).
Bertrand, C., Bergounioux, C., Domenichini, S., Delarue, M. & Zhou, D. X. Arabidopsis histone acetyltransferase AtGCN5 regulates the floral meristem activity through the WUSCHEL/AGAMOUS pathway. J. Biol. Chem. 278, 28246–28251 (2003).
Kuzmichev, A., Nishioka, K., Erdjument-Bromage, H., Tempst, P. & Reinberg, D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 16, 2893–2905 (2002).
Calonje, M., Sanchez, R., Chen, L. & Sung, Z. R. EMBRYONIC FLOWER1 participates in Polycomb group-mediated AG gene silencing in Arabidopsis. Plant Cell 20, 277–291 (2008).
Schubert, D. et al. Silencing by plant Polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. EMBO J. 25, 4638–4649 (2006).
Aichinger, E. et al. CHD3 proteins and Polycomb group proteins antagonistically determine cell identity in Arabidopsis. PLOS Genet. 5, e1000605 (2009).
Chanvivattana, Y. et al. Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development 131, 5263–5276 (2004).
Adrian, J., Torti, S. & Turck, F. From decision to commitment: the molecular memory of flowering. Mol. Plant 2, 628–642 (2009).
Poux, S., Horard, B., Sigrist, C. J. A. & Pirrotta, V. The Drosophila Trithorax protein is a coactivator required to prevent reestablishment of Polycomb silencing. Development 129, 2483–2493 (2002).
Klymenko, T. & Muller, J. The histone methyltransferases Trithorax and Ash1 prevent transcriptional silencing by Polycomb group proteins. EMBO Reports 5, 373–377 (2004).
Zhang, X. Y., Bernatavichute, Y. V., Cokus, S., Pellegrini, M. & Jacobsen, S. E. Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol. 10, R62 (2009).
Alvarez-Venegas, R. & Avramova, Z. Two Arabidopsis homologs of the animal trithorax genes: a new structural domain is a signature feature of the trithorax gene family. Gene 271, 215–221 (2001).
Tamada, Y., Yun, J. Y., Woo, S. C. & Amasino, R. M. Arabidopsis TRITHORAX-RELATED7 is required for methylation of lysine 4 of histone H3 and for transcriptional activation of FLOWERING LOCUS C. Plant Cell 21, 3257–3269 (2009).
Saleh, A. et al. The highly similar Arabidopsis homologs of Trithorax ATX1 and ATX2 encode proteins with divergent biochemical functions. Plant Cell 20, 568–579 (2008).
Carles, C. C. & Fletcher, J. C. The SAND domain protein ULTRAPETALA1 acts as a Trithorax group factor to regulate cell fate in plants. Genes Dev. 23, 2723–2728 (2009).
De Lucia, F., Crevillen, P., Jones, A. M. E., Greb, T. & Dean, C. A PHD–polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc. Natl. Acad. Sci. U.S.A. 105, 16831–16836 (2008). This publication describes the first liquid chromatography–mass spectrometry-based identification of a PcG protein complex in plants. It also shows how addition of individual subunits in response to an environmental signal can drastically affect activity of the complex at the FLC locus.
Vielle-Calzada, J. P. et al. Maintenance of genomic imprinting at the Arabidopsis medea locus requires zygotic DDM1 activity. Genes Dev. 13, 2971–2982 (1999).
Schwartz, Y. B. & Pirrotta, V. Polycomb silencing mechanisms and the management of genomic programmes. Nature Rev. Genet. 8, 9–22 (2007).
Henderson, I. R. & Jacobsen, S. E. Epigenetic inheritance in plants. Nature 447, 418–424 (2007).
Zhang, X. Y. et al. Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLOS Biol. 5, 1026–1035 (2007).
Liu, C., Xi, W. Y., Shen, L. S., Tan, C. P. & Yu, H. Regulation of floral patterning by flowering time genes. Dev. Cell 16, 711–722 (2009). This paper describes the direct interaction between transcription factors and components of histone-modifying protein complexes, and their role, which is to prevent the precocious activation of floral organ identity genes in floral meristems.
Hill, K., Wang, H. & Perry, S. E. A transcriptional repression motif in the MADS factor AGL15 is involved in recruitment of histone deacetylase complex components. Plant J. 53, 172–185 (2008).
de Folter, S. et al. Comprehensive interaction map of the Arabidopsis MADS box transcription factors. Plant Cell 17, 1424–1433 (2005).
Sridhar, V. V., Surendrarao, A. & Liu, Z. C. APETALA1 and SEPALLATA3 interact with SEUSS to mediate transcription repression during flower development. Development 133, 3159 (2006). This publication describes the interaction, mediated by SEU, of MADS-domain transcription factors that control floral meristem and organ identity with the Groucho-type transcriptional co-repressor LUG. It also indicates a role for this interaction in the repression of AG expression in the outer floral whorls.
Franks, R. G., Wang, C. X., Levin, J. Z. & Liu, Z. C. SEUSS, a member of a novel family of plant regulatory proteins, represses floral homeotic gene expression with LEUNIG. Development 129, 253–263 (2002).
Conner, J. & Liu, Z. C. LEUNIG, a putative transcriptional corepressor that regulates AGAMOUS expression during flower development. Proc. Natl. Acad. Sci. U.S.A. 97, 12902–12907 (2000).
Liu, Z. C. & Karmarkar, V. Groucho/Tup1 family co-repressors in plant development. Trends Plant Sci. 13, 137–144 (2008).
Gonzalez, D., Bowen, A. J., Carroll, T. S. & Conlan, R. S. The transcription corepressor LEUNIG interacts with the histone deacetylase HDA19 and Mediator components MED14 (SWP) and CDK8 (HEN3) to repress transcription. Mol. Cell. Biol. 27, 5306–5315 (2007).
Perissi, V., Jepsen, K., Glass, C. K. & Rosenfeld, M. G. Deconstructing repression: evolving models of co-repressor action. Nature Rev. Genet. 11, 109–123 (2010).
Navarro, C. et al. Molecular and genetic interactions between STYLOSA and GRAMINIFOLIA in the control of Antirrhinum vegetative and reproductive development. Development 131, 3649–3659 (2004).
Stahle, M. I., Kuehlich, J., Staron, L., von Arnim, A. G. & Golz, J. F. YABBYs and the transcriptional corepressors LEUNIG and LEUNIG_HOMOLOG maintain leaf polarity and meristem activity in Arabidopsis. Plant Cell 21, 3105–3118 (2009).
Szemenyei, H., Hannon, M. & Long, J. A. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319, 1384–1386 (2008).
Long, J. A., Ohno, C., Smith, Z. R. & Meyerowitz, E. M. TOPLESS regulates apical embryonic fate in Arabidopsis. Science 312, 1520–1523 (2006).
Sun, B., Xu, Y. F., Ng, K. H. & Ito, T. A timing mechanism for stem cell maintenance and differentiation in the Arabidopsis floral meristem. Genes Dev. 23, 1791–1804 (2009). This publication describes the timed activation of the WUS repressor KNUCKLES by AG, which is required for coordinated stem cell termination in the floral meristem. It provides an example of the interplay between transcription factor-mediated and epigenetic regulation of activation of gene expression during development.
Adrian, J. et al. Cis-regulatory elements and chromatin state coordinately control temporal and spatial expression of FLOWERING LOCUS T in Arabidopsis. Plant Cell 22, 1425–1440 (2010).
Geisberg, J. V. & Struhl, K. Quantitative sequential chromatin immunoprecipitation, a method for analyzing co-occupancy of proteins at genomic regions in vivo. Nucleic Acids Res. 32, e151 (2004).
Deal, R. B. & Henikoff, S. A simple method for gene expression and chromatin profiling of individual cell types within a tissue. Dev. Cell 18, 1030–1040 (2010).
Jiao, Y. & Meyerowitz, E. M. Cell-type specific analysis of translating RNAs in developing flowers reveals new levels of control. Mol. Syst. Biol. 6, 419 (2010).
Louwers, M., Splinter, E., van Driel, R., de Laat, W. & Stam, M. Studying physical chromatin interactions in plants using chromosome conformation capture (3C). Nature Protoc. 4, 1216–1229 (2009).
Visel, A., Rubin, E. M. & Pennacchio, L. A. Genomic views of distant-acting enhancers. Nature 461, 199–205 (2009).
Zheng, Y., Ren, N., Wang, H., Stromberg, A. J. & Perry, S. E. Global identification of targets of the Arabidopsis MADS domain protein AGAMOUS-like15. Plant Cell 21, 2563–2577 (2009).
Gertz, J., Siggia, E. D. & Cohen, B. A. Analysis of combinatorial cis-regulation in synthetic and genomic promoters. Nature 457, U215–U113 (2009).
Segal, E. & Widom, J. From DNA sequence to transcriptional behaviour: a quantitative approach. Nature Rev. Genet. 10, 443–456 (2009).
Lanzuolo, C., Roure, V., Dekker, J., Bantignies, F. & Orlando, V. Polycomb response elements mediate the formation of chromosome higher-order structures in the bithorax complex. Nature Cell Biol. 9, 1167–1174 (2007).
Fraser, P. & Bickmore, W. Nuclear organization of the genome and the potential for gene regulation. Nature 447, 413–417 (2007).
Cook, P. R. A model for all genomes: the role of transcription factories. J. Mol. Biol. 395, 1–10 (2010).
Egea-Cortines, M., Saedler, H. & Sommer, H. Ternary complex formation between the MADS-box proteins SQUAMOSA, DEFICIENS and GLOBOSA is involved in the control of floral architecture in Antirrhinum majus. EMBO J. 18, 5370–5379 (1999).
Melzer, R., Verelst, W. & Theissen, G. The class E floral homeotic protein SEPALLATA3 is sufficient to loop DNA in floral quartet-like complexes in vitro. Nucleic Acids Res. 37, 144–157 (2009).
Rippe, K. Making contacts on a nucleic acid polymer. Trends Biochem. Sci. 26, 733–740 (2001).
Riechmann, J. L., Wang, M. Q. & Meyerowitz, E. M. DNA-binding properties of Arabidopsis MADS domain homeotic proteins APETALA1, APETALA3, PISTILLATA and AGAMOUS. Nucleic Acids Res. 24, 3134–3141 (1996).
West, A. G., Causier, B. E., Davies, B. & Sharrocks, A. D. DNA binding and dimerisation determinants of Antirrhinum majus MADS-box transcription factors. Nucleic Acids Res. 26, 5277–5287 (1998).
Chuck, G., Cigan, A. M., Saeteurn, K. & Hake, S. The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nature Genet. 39, 544–549 (2007).
Wu, G. et al. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138, 750–759 (2009).
Yamaguchi, A. et al. The microRNA-regulated SBP-box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev. Cell 17, 268–278 (2009). References 32, 149 and 150 describe the sequential action of miR156 and miR172 in controlling major phase changes in Arabidopsis development.
Emery, J. F. et al. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 13, 1768–1774 (2003).
Williams, L., Grigg, S. P., Xie, M. T., Christensen, S. & Fletcher, J. C. Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes. Development 132, 3657–3668 (2005).
Carlsbecker, A. et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 465, 316–321 (2010).
Scheres, B. Developmental biology: roots respond to an inner calling. Nature 465, 299–300 (2010).
Laufs, P., Peaucelle, A., Morin, H. & Traas, J. MicroRNA regulation of the CUC genes is required for boundary size control in Arabidopsis meristems. Development 131, 4311–4322 (2004).
Mallory, A. C., Dugas, D. V., Bartel, D. P. & Bartel, B. MicroRNA regulation of NAC-domain targets is required for proper formation and separation of adjacent embryonic, vegetative, and floral organs. Curr. Biol. 14, 1035–1046 (2004).
Wollmann, H., Mica, E., Todesco, M., Long, J. A. & Weigel, D. On reconciling the interactions between APETALA2, miR172 and AGAMOUS with the ABC model of flower development. Development 137, 3633–3642 (2010).
Cartolano, M. et al. A conserved microRNA module exerts homeotic control over Petunia hybrida and Antirrhinum majus floral organ identity. Nature Genet. 39, 901–905 (2007).
Nag, A. & Jack, T. Sculpting the flower; the role of MICRORNAs in flower development. Plant Dev. 91, 349–378 (2010).
Chen, X. M. Small RNAs and their roles in plant development. Annu. Rev. Cell Dev. Biol. 25, 21–44 (2009).
Sieburth, L. E. & Meyerowitz, E. M. Molecular dissection of the AGAMOUS control region shows that cis elements for spatial regulation are located intragenically. Plant Cell 9, 355–365 (1997).
Deyholos, M. K. & Sieburth, L. E. Separable whorl-specific expression and negative regulation by enhancer elements within the AGAMOUS second intron. Plant Cell 12, 1799–1810 (2000).
Gaudin, V. et al. Mutations in LIKE HETEROCHROMATIN PROTEIN 1 affect flowering time and plant architecture in Arabidopsis. Development 128, 4847–4858 (2001).
Turck, F. et al. Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLOS Genet. 3, 855–866 (2007).
Bao, X. Z., Franks, R. G., Levin, J. Z. & Liu, Z. C. Repression of AGAMOUS by BELLRINGER in floral and inflorescence meristems. Plant Cell 16, 1478–1489 (2004).
Maier, A. T. et al. Dual roles of the bZIP transcription factor PERIANTHIA in the control of floral architecture and homeotic gene expression. Development 136, 1613–1620 (2009).
Das, P. et al. Floral stem cell termination involves the direct regulation of AGAMOUS by PERIANTHIA. Development 136, 1605–1611 (2009).
Lohmann, J. U. et al. A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105, 793–803 (2001).
Willmann, M. R. & Poethig, R. S. Time to grow up: the temporal role of small RNAs in plants. Curr. Opin. Plant Biol. 8, 548–552 (2005).
Samach, A. et al. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288, 1613–1616 (2000).
Corbesier, L. et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316, 1030–1033 (2007).
Chae, E., Tan, Q. K., Hill, T. A. & Irish, V. F. An Arabidopsis F-box protein acts as a transcriptional co-factor to regulate floral development. Development 135, 1235–1245 (2008).
Fornara, F., de Montaigu, A. & Coupland, G. Control of flowering in Arabidopsis. Cell 141, 550–550.e2 (2010).
Acknowledgements
We thank R. de Maagd, J. M. Muino, R. Karlova, J. Wellink and R. Immink for useful comments on the manuscript. We wish to apologize to all authors whose publications we could not cite due to space constraints.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Examples of genome-wide target gene studies of transcription factors controlling development in Arabidopsis thaliana. (PDF 343 kb)
Supplementary information S2 (table)
Examples of chromatin modifying and remodeling complexes with roles in developmental switches in plants (PDF 364 kb)
Related links
Glossary
- Pluripotent cell
-
An undifferentiated cell that has the potential to adopt different identities. In plants, pluripotent cells are found in meristems and there are stem cell-like populations in shoots, roots and leaves.
- Meristem
-
A tissue in plants consisting of pluripotent cells. In apical meristems, cell-to-cell signalling establishes and maintains a zone that contains the stem cells, which is separated from the peripheral zone in which differentiation is eventually initiated. Other types of meristems give rise to the vasaculture and epidermis, or enable secondary growth.
- Shoot apical meristem
-
The meristem that forms all major above-ground plant organs. It is established during embryogenesis. During plant development, it changes from a juvenile to a vegetative state, and then to inflorescence and floral identity.
- Inflorescence meristem
-
A type of shoot apical meristem that gives rise to floral meristems at its flanks.
- Floral meristem
-
A meristem that gives rise to the floral organs: sepals, petals, stamens and carpels.
- Histone-modifying enzyme
-
An enzyme that can modify specific sites in histones, for example, by adding or removing a chemical group. Common modifications are methylation, acetylation, ubiquitylation, sumoylation, phosphorylation and proline isomerization.
- Nucleosome-remodelling enzyme
-
An enzyme that can establish, remove, or change the positions of nucleosomes on the DNA.
- MADS-box family
-
A family of transcription factors that is present in all major groups of eukaryotes. The family is named after the founding members MCM1 from Saccharomyces cerevisiae, AGAMOUS from Arabidopsis thaliana, DEFICIENS from Antirrhinum majus and SRF from humans.
- ChIP–seq
-
(Chromatin immunoprecipitation followed by next-generation sequencing). A technique that is used to identify the in vivo DNA-binding sites of proteins. After crosslinking of proteins to DNA, isolation and fragmentation of the chromatin, genomic regions that are bound by the protein of interest are isolated using specific antibodies. The immunoprecipitated DNA is then sequenced.
- ChIP–chip
-
(Chromatin immunoprecipitation followed by microarray). DNA associated with a protein of interest, isolated by chromatin immunoprecipitation, is hybridized to genomic-tiling arrays to identify DNA-binding sites of the protein.
- Direct target gene
-
A gene whose expression is controlled by a particular transcription factor through direct binding of the factor to cis-regulatory elements of that gene.
- Autoregulation
-
A mechanism in which a molecule (such as a transcription factor) regulates its own production. This process can involve interactions with other molecules.
- Floral pathway integrator
-
A protein that can integrate the inputs of the different environmental and internal floral induction pathways and transmit the information to their downstream targets, such as floral meristem identity genes, at the shoot apex. Their combined action controls flowering time. The transcriptional regulators SOC1, LFY, FT and FD are 'classical' floral pathway integrators.
- Perianth
-
The sterile organs in the outer whorls of a flower. Many flowering plant species such as Arabidopsis, Petunia and Antirrhinum have a perianth that is differentiated into sepals and petals.
- Trichome
-
An epidermal outgrowth (hair) that can have different structures and functions. In Arabidopsis thaliana, trichomes are unicellular.
- Stoma
-
A pore found in the epidermis of leaves and in several other above-ground plant organs. Stomata are surrounded by pairs of specialized epidermal cells called guard cells.
- Homeotic mutant
-
A mutant in which one organ type is replaced by a different organ type.
- Stele
-
The central part of the root, mainly consisting of the vasculature and the surrounding pericycle.
- Floral reversion
-
The reversion of a meristem from a reproductive state back to a vegetative state, caused by mutations in regulatory genes. A floral meristem can revert to an inflorescence meristem or an inflorescence meristem can revert to a vegetative meristem. Reversion leads to the formation of shoots instead of flowers and 'aerial' rosettes instead of shoots.
- (CHD)-type nucleosome remodeller
-
An ATP-dependent chromatin-remodelling factor of the chromodomain/helicase/DNA-binding domain (CHD) subfamily. These remodellers usually function as part of multisubunit complexes. In mammals and flies, they are involved in transcriptional repression by nucleosome remodelling and histone deacetylation. They have also been shown to be involved in transcriptional activation.
- Vernalization
-
The induction of the transition from vegetative to reproductive plant growth by a prolonged period of cold (winter).
- Sequential ChIP
-
The identification of DNA-binding sites that are common to two proteins (for example, two types of modified histones or transcription factors). It involves two rounds of immunoprecipitation using separate antibodies against the proteins of interest.
Rights and permissions
About this article
Cite this article
Kaufmann, K., Pajoro, A. & Angenent, G. Regulation of transcription in plants: mechanisms controlling developmental switches. Nat Rev Genet 11, 830–842 (2010). https://doi.org/10.1038/nrg2885
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg2885
This article is cited by
-
One pattern analysis (OPA) for the quantitative determination of protein interactions in plant cells
Plant Methods (2023)
-
3D organization of regulatory elements for transcriptional regulation in Arabidopsis
Genome Biology (2023)
-
The RNA polymerase II subunit NRPB2 is required for indeterminate root development, cell viability, stem cell niche maintenance, and de novo root tip regeneration in Arabidopsis
Protoplasma (2022)
-
Balancing yield trade-off in legumes during multiple stress tolerance via strategic crosstalk by native NAC transcription factors
Journal of Plant Biochemistry and Biotechnology (2021)
-
Genome-wide MNase hypersensitivity assay unveils distinct classes of open chromatin associated with H3K27me3 and DNA methylation in Arabidopsis thaliana
Genome Biology (2020)