Key Points
-
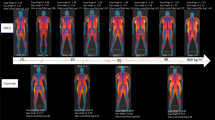
Congenital generalized lipodystrophy (CGL) is a heterogeneous autosomal recessive disorder characterized by near total absence of body fat and extreme muscularity present at birth or soon thereafter
-
Patients with CGL are extremely hypoleptinaemic and are predisposed to develop metabolic complications, such as diabetes mellitus, hypertriglyceridaemia and hepatic steatosis
-
Four distinct subtypes of CGL exist: type 1 is associated with AGPAT2 mutations; type 2 is associated with BSCL2 mutations; type 3 is associated with CAV1 mutation; and type 4 is associated with PTRF mutations
-
Therapeutic options for patients with CGL include conventional lipid-lowering and antihyperglycaemic drugs, as well as metreleptin replacement therapy
Abstract
Congenital generalized lipodystrophy (CGL) is a heterogeneous autosomal recessive disorder characterized by a near complete lack of adipose tissue from birth and, later in life, the development of metabolic complications, such as diabetes mellitus, hypertriglyceridaemia and hepatic steatosis. Four distinct subtypes of CGL exist: type 1 is associated with AGPAT2 mutations; type 2 is associated with BSCL2 mutations; type 3 is associated with CAV1 mutations; and type 4 is associated with PTRF mutations. The products of these genes have crucial roles in phospholipid and triglyceride synthesis, as well as in the formation of lipid droplets and caveolae within adipocytes. The predominant cause of metabolic complications in CGL is excess triglyceride accumulation in the liver and skeletal muscle owing to the inability to store triglycerides in adipose tissue. Profound hypoleptinaemia further exacerbates metabolic derangements by inducing a voracious appetite. Patients require psychological support, a low-fat diet, increased physical activity and cosmetic surgery. Aside from conventional therapy for hyperlipidaemia and diabetes mellitus, metreleptin replacement therapy can dramatically improve metabolic complications in patients with CGL. In this Review, we discuss the molecular genetic basis of CGL, the pathogenesis of the disease's metabolic complications and therapeutic options for patients with CGL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Garg, A. Acquired and inherited lipodystrophies. N. Engl. J. Med. 350, 1220–1234 (2004).
Berardinelli, W. An undiagnosed endocrinometabolic syndrome: report of 2 cases. J. Clin. Endocrinol. Metab. 14, 193–204 (1954).
Seip, M. Lipodystrophy and gigantism with associated endocrine manifestations: a new diencephalic syndrome? Acta Paediatrica 48, 555–574 (1959).
Wiedemann, H. R. Newly recognized congenital progeroid disorder. Am. J. Med. Genet. 42, 857 (1992).
Rautenstrauch, T., Snigula, F. & Wiedemann, H. R. Neonatal progeroid syndrome (Wiedemann-Rautenstrauch). A follow-up study [German]. Klin. Padiatr. 206, 440–443 (1994).
Dunnigan, M. G., Cochrane, M. A., Kelly, A. & Scott, J. W. Familial lipoatrophic diabetes with dominant transmission. A new syndrome. Q. J. Med. 43, 33–48 (1974).
Young, L. W. et al. New syndrome manifested by mandibular hypoplasia, acroosteolysis, stiff joints and cutaneous atrophy (mandibuloacral dysplasia) in two unrelated boys. Birth Defects Orig. Artic. Ser. 7, 291–297 (1971).
Eriksson, M. et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, 293–298 (2003).
De Sandre-Giovannoli, A. et al. Lamin A truncation in Hutchinson-Gilford progeria. Science 300, 2055 (2003).
Garg, A. et al. Atypical progeroid syndrome due to heterozygous missense LMNA mutations. J. Clin. Endocrinol. Metab. 94, 4971–4983 (2009).
Pelosini, C. et al. Identification of a novel mutation in the polymerase delta 1 (POLD1) gene in a lipodystrophic patient affected by mandibular hypoplasia, deafness, progeroid features (MDPL) syndrome. Metabolism 63, 1385–1389 (2014).
Capeau, J. et al. Human lipodystrophies: genetic and acquired diseases of adipose tissue. Endocr. Dev. 19, 1–20 (2010).
Agarwal, A. K. et al. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J. Clin. Endocrinol. Metab. 88, 4840–4847 (2003).
Chan, J. L. & Oral, E. A. Clinical classification and treatment of congenital and acquired lipodystrophy. Endocr. Pract. 16, 310–323 (2010).
Van Maldergem, L. et al. Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J. Med. Genet. 39, 722–733 (2002).
Thauvin-Robinet, C. et al. PIK3R1 mutations cause syndromic insulin resistance with lipoatrophy. Am. J. Hum. Genet. 93, 141–149 (2013).
Dyment, D. A. et al. Mutations in PIK3R1 cause SHORT syndrome. Am. J. Hum. Genet. 93, 158–166 (2013).
Chudasama, K. K. et al. SHORT syndrome with partial lipodystrophy due to impaired phosphatidylinositol 3 kinase signaling. Am. J. Hum. Genet. 93, 150–157 (2013).
Masotti, A. et al. Keppen-Lubinsky syndrome is caused by mutations in the inwardly rectifying K+ channel encoded by KCNJ6. Am. J. Hum. Genet. 96, 295–300 (2015).
Shastry, S. et al. A novel syndrome of mandibular hypoplasia, deafness, and progeroid features associated with lipodystrophy, undescended testes, and male hypogonadism. J. Clin. Endocrinol. Metab. 95, E192–E197 (2010).
Garg, A. & Xing, C. De novo heterozygous FBN1 mutations in the extreme C-terminal region cause progeroid fibrillinopathy. Am. J. Med. Genet. A 164A, 1341–1345 (2014).
Garg, A. et al. Whole exome sequencing identifies de novo heterozygous CAV1 mutations associated with a novel neonatal onset lipodystrophy syndrome. Am. J. Med. Genet. A http://dx.doi.org/10.1002/ajmg.a.37115.
Prokocimer, M., Barkan, R. & Gruenbaum, Y. Hutchinson-Gilford progeria syndrome through the lens of transcription. Aging Cell 12, 533–543 (2013).
Ahmad, Z., Zackai, E., Medne, L. & Garg, A. Early onset mandibuloacral dysplasia due to compound heterozygous mutations in ZMPSTE24. Am. J. Med. Genet. A 152A, 2703–2710 (2010).
Simha, V., Agarwal, A. K., Oral, E. A., Fryns, J. P. & Garg, A. Genetic and phenotypic heterogeneity in patients with mandibuloacral dysplasia-associated lipodystrophy. J. Clin. Endocrinol. Metab. 88, 2821–2824 (2003).
Agarwal, A. K. et al. PSMB8 encoding the β5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am. J. Hum. Genet. 87, 866–872 (2010).
Farhan, S. M. et al. A novel LIPE nonsense mutation found using exome sequencing in siblings with late-onset familial partial lipodystrophy. Can. J. Cardiol. 30, 1649–1654 (2014).
Saha, B. et al. A novel LMNA mutation causes altered nuclear morphology and symptoms of familial partial lipodystrophy (Dunnigan variety) with progeroid features. Mol. Syndromol. 1, 127–132 (2010).
Donadille, B. et al. Partial lipodystrophy with severe insulin resistance and adult progeria Werner syndrome. Orphanet J. Rare Dis. 8, 106 (2013).
Payne, F. et al. Mutations disrupting the Kennedy phosphatidylcholine pathway in humans with congenital lipodystrophy and fatty liver disease. Proc. Natl Acad. Sci. USA 111, 8901–8906 (2014).
Garg, A. Lipodystrophies. Am. J. Med. 108, 143–152 (2000).
Garg, A. Clinical review: Lipodystrophies: genetic and acquired body fat disorders. J. Clin. Endocrinol. Metab. 96, 3313–3325 (2011).
Nolis, T. Exploring the pathophysiology behind the more common genetic and acquired lipodystrophies. J. Hum. Genet. 59, 16–23 (2014).
Pardini, V. C. et al. Leptin levels, β-cell function, and insulin sensitivity in families with congenital and acquired generalized lipoatrophic diabetes. J. Clin. Endocrinol. Metab. 83, 503–508 (1998).
Magre, J. et al. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat. Genet. 28, 365–370 (2001).
Agarwal, A. K. et al. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat. Genet. 31, 21–23 (2002).
Kim, C. A. et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J. Clin. Endocrinol. Metab. 93, 1129–1134 (2008).
Hayashi, Y. K. et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J. Clin. Invest. 119, 2623–2633 (2009).
Dyment, D. A. et al. Biallelic mutations at PPARG cause a congenital, generalized lipodystrophy similar to the Berardinelli-Seip syndrome. Eur. J. Med. Genet. 57, 524–526 (2014).
Knebel, B. et al. A mutation in the c-fos gene associated with congenital generalized lipodystrophy. Orphanet J. Rare Dis. 8, 119 (2013).
Seip, M. & Trygstad, O. Generalized lipodystrophy, congenital and acquired (lipoatrophy). Acta Paediatrica Suppl. 413, 2–28 (1996).
Westvik, J. Radiological features in generalized lipodystrophy. Acta Paediatrica Suppl. 413, 44–51 (1996).
Garg, A., Fleckenstein, J. L., Peshock, R. M. & Grundy, S. M. Peculiar distribution of adipose tissue in patients with congenital generalized lipodystrophy. J. Clin. Endocrinol. Metab. 75, 358–361 (1992).
Seip, M. & Trygstad, O. Generalized lipodystrophy, congenital and acquired (lipoatrophy). Acta Paediatr. Suppl. 413, 2–28 (1996).
Garg, A. et al. A gene for congenital generalized lipodystrophy maps to human chromosome 9q34. J. Clin. Endocrinol. Metab. 84, 3390–3394 (1999).
[No authors listed]. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 1–1975. N. Engl. J. Med. 292, 35–41 (1975).
Chandalia, M., Garg, A., Vuitch, F. & Nizzi, F. Postmortem findings in congenital generalized lipodystrophy. J. Clin. Endocrinol. Metab. 80, 3077–3081 (1995).
Javor, E. D. et al. Proteinuric nephropathy in acquired and congenital generalized lipodystrophy: baseline characteristics and course during recombinant leptin therapy. J. Clin. Endocrinol. Metab. 89, 3199–3207 (2004).
Upreti, V., Dhull, P., Patnaik, S. K. & Kumar, K. V. An unusual cause of delayed puberty: Berardinelli-Seip syndrome. J. Pediatr. Endocrinol. Metab. 25, 1157–1160 (2012).
Musso, C. et al. The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism 54, 255–263 (2005).
Lungu, A. O., Zadeh, E. S., Goodling, A., Cochran, E. & Gorden, P. Insulin resistance is a sufficient basis for hyperandrogenism in lipodystrophic women with polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 97, 563–567 (2012).
Abel, B. S., Muniyappa, R., Skarulis, M. C., Gorden, P. & Brown, R. J. Recombinant human leptin (metreleptin) administration augments nocturnal LH secretion in lipodystrophy patients. Presented at The Endocrine Society's 97th Annual Meeting & Expo (2015).
Maguire, M., Lungu, A., Gorden, P., Cochran, E. & Stratton, P. Pregnancy in a woman with congenital generalized lipodystrophy: leptin's vital role in reproduction. Obstet. Gynecol. 119, 452–455 (2012).
McNally, M. et al. Successful renal transplantation in a patient with congenital generalized lipodystrophy: a case report. Am. J. Transplant. 4, 447–449 (2004).
Miranda, D. M. et al. Novel mutations of the BSCL2 and AGPAT2 genes in 10 families with Berardinelli-Seip congenital generalized lipodystrophy syndrome. Clin. Endocrinol. (Oxf.) 71, 512–517 (2009).
Gomes, K. B. et al. Mutations in the Seipin and AGPAT2 genes clustering in consanguineous families with Berardinelli-Seip congenital lipodystrophy from two separate geographical regions of Brazil. J. Clin. Endocrinol. Metab. 89, 357–361 (2004).
Jiang, M. et al. Lack of testicular seipin causes teratozoospermia syndrome in men. Proc. Natl Acad. Sci. USA 111, 7054–7059 (2014).
Brunzell, J. D., Shankle, S. W. & Bethune, J. E. Congenital generalized lipodystrophy accompanied by cystic angiomatosis. Ann. Intern. Med. 69, 501–516 (1968).
Fleckenstein, J. L., Garg, A., Bonte, F. J., Vuitch, M. F. & Peshock, R. M. The skeleton in congenital, generalized lipodystrophy: evaluation using whole-body radiographic surveys, magnetic resonance imaging and technetium-99m bone scintigraphy. Skeletal Radiol. 21, 381–386 (1992).
Guell-Gonzalez, J. R. et al. Bone lesions in congenital generalised lipodystrophy. Lancet 2, 104–105 (1971).
Shinya, T. et al. Computed tomography findings of congenital generalized lipodystrophy: multiple nodular fatty liver and diffuse sclerosis of bones. Radiat. Med. 25, 484–487 (2007).
Shirwalkar, H. U. et al. Congenital generalized lipodystrophy in an Indian patient with a novel mutation in BSCL2 gene. J. Inherit Metab. Dis. 31 (Suppl. 2), S317–S322 (2008).
Christensen, J. D. et al. Bone mineral content in patients with congenital generalized lipodystrophy is unaffected by metreleptin replacement therapy. J. Clin. Endocrinol. Metab. 99, E1493–E1500 (2014).
Garg, A., Chandalia, M. & Vuitch, F. Severe islet amyloidosis in congenital generalized lipodystrophy. Diabetes Care 19, 28–31 (1996).
Oral, E. A. et al. Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 346, 570–578 (2002).
Diker-Cohen, T., Cochran, E., Gorden, P. & Brown, R. J. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J. Clin. Endocrinol. Metab. 100, 1802–1810 (2015).
Garg, A. in Dyslipidemias: Pathophysiology, Evaluation and Management. (ed. Garg, A.) 287–302 (Springer, 2015).
Rego, A. G. et al. Cardiometabolic abnormalities in patients with Berardinelli-Seip syndrome [Portuguese]. Arq. Bras. Cardiol. 94, 109–118 (2010).
Indumathi, C. K., Lewin, S. & Ayyar, V. Berardinelli Seip syndrome with insulin-resistant diabetes mellitus and stroke in an infant. Indian J. Endocrinol. Metab. 15, S62–S64 (2011).
Haque, W. A., Shimomura, I., Matsuzawa, Y. & Garg, A. Serum adiponectin and leptin levels in patients with lipodystrophies. J. Clin. Endocrinol. Metab. 87, 2395–2398 (2002).
Antuna-Puente, B. et al. Higher adiponectin levels in patients with Berardinelli-Seip congenital lipodystrophy due to seipin as compared with 1-acylglycerol-3-phosphate-o-acyltransferase-2 deficiency. J. Clin. Endocrinol. Metab. 95, 1463–1468 (2010).
McDuffie, J. R. et al. Effects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiency. J. Clin. Endocrinol. Metab. 89, 4258–4263 (2004).
Rajab, A. et al. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet. 6, e1000874 (2010).
Shastry, S. et al. Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am. J. Med. Genet. A 152A, 2245–2253 (2010).
Ardissone, A. et al. Novel PTRF mutation in a child with mild myopathy and very mild congenital lipodystrophy. BMC Med. Genet. 14, 89 (2013).
Dwianingsih, E. K. et al. A Japanese child with asymptomatic elevation of serum creatine kinase shows PTRF-CAVIN mutation matching with congenital generalized lipodystrophy type 4. Mol. Genet. Metab. 101, 233–237 (2010).
UniProt. FOS - FOS Protein - Homo sapiens (human) [online], (2015).
Online Mendelian Inheritance in Man©. #608594 Lipodystrophy, congenital generalized, type 1; CGL1 [online], (2014).
Garg, A. & Agarwal, A. K. Lipodystrophies: disorders of adipose tissue biology. Biochim. Biophys. Acta 1791, 507–513 (2009).
Cortes, V. A. et al. Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell Metab. 9, 165–176 (2009).
Agarwal, A. K. & Garg, A. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol. Metab. 14, 214–221 (2003).
Takeuchi, K. & Reue, K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 296, E1195–E1209 (2009).
Leung, D. W. The structure and functions of human lysophosphatidic acid acyltransferases. Front. Biosci. 6, D944–D953 (2001).
Agarwal, A. K., Barnes, R. I. & Garg, A. Genetic basis of congenital generalized lipodystrophy. Int. J. Obes. Relat. Metab. Disord. 28, 336–339 (2004).
Agarwal, A. K. & Garg, A. Enzymatic activity of the human 1-acylglycerol-3-phosphate-O-acyltransferase isoform 11: upregulated in breast and cervical cancers. J. Lipid Res. 51, 2143–2152 (2010).
Agarwal, A. K. Lysophospholipid acyltransferases: 1-acylglycerol-3-phosphate O-acyltransferases. From discovery to disease. Curr. Opin. Lipidol. 23, 290–302 (2012).
Fu, M. et al. Mutations in Gng3lg and AGPAT2 in Berardinelli-Seip congenital lipodystrophy and Brunzell syndrome: phenotype variability suggests important modifier effects. J. Clin. Endocrinol. Metab. 89, 2916–2922 (2004).
Magre, J. et al. Prevalence of mutations in AGPAT2 among human lipodystrophies. Diabetes 52, 1573–1578 (2003).
Haque, W., Garg, A. & Agarwal, A. K. Enzymatic activity of naturally occurring 1-acylglycerol-3-phosphate-O-acyltransferase 2 mutants associated with congenital generalized lipodystrophy. Biochem. Biophys. Res. Commun. 327, 446–453 (2005).
Simha, V. & Garg, A. Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy due to mutations in the AGPAT2 or Seipin genes. J. Clin. Endocrinol. Metab. 88, 5433–5437 (2003).
Gale, S. E. et al. A regulatory role for 1-acylglycerol-3-phosphate-O-acyltransferase 2 in adipocyte differentiation. J. Biol. Chem. 281, 11082–11089 (2006).
Subauste, A. R. et al. Alterations in lipid signaling underlie lipodystrophy secondary to AGPAT2 mutations. Diabetes 61, 2922–2931 (2012).
Online Mendelian Inheritance in Man©. #269700 Lipodystrophy, congenital generalized, type 2; CGL2 [online], (2014).
Agarwal, A. K. & Garg, A. Seipin: a mysterious protein. Trends Mol. Med. 10, 440–444 (2004).
Szymanski, K. M. et al. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc. Natl Acad. Sci. USA 104, 20890–20895 (2007).
Fei, W. et al. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J. Cell Biol. 180, 473–482 (2008).
Yang, W. et al. BSCL2/seipin regulates adipogenesis through actin cytoskeleton remodelling. Hum. Mol. Genet. 23, 502–513 (2014).
Cartwright, B. R. et al. Seipin performs dissectible functions in promoting lipid droplet biogenesis and regulating droplet morphology. Mol. Biol. Cell 26, 726–739 (2015).
Talukder, M. M., Sim, M. F., O'Rahilly, S., Edwardson, J. M. & Rochford, J. J. Seipin oligomers can interact directly with AGPAT2 and lipin 1, physically scaffolding critical regulators of adipogenesis. Mol. Metab. 4, 199–209 (2015).
Sim, M. F. et al. Analysis of naturally occurring mutations in the human lipodystrophy protein seipin reveals multiple potential pathogenic mechanisms. Diabetologia 56, 2498–2506 (2013).
Sim, M. F. et al. The human lipodystrophy protein seipin is an ER membrane adaptor for the adipogenic PA phosphatase lipin 1. Mol. Metab. 2, 38–46 (2012).
Wee, K., Yang, W., Sugii, S. & Han, W. Towards a mechanistic understanding of lipodystrophy and seipin functions. Biosci. Rep. 3, e00141 (2014).
Schuster, J. et al. Exome sequencing circumvents missing clinical data and identifies a BSCL2 mutation in congenital lipodystrophy. BMC Med. Genet. 15, 71 (2014).
Guillen-Navarro, E. et al. A new seipin-associated neurodegenerative syndrome. J. Med. Genet. 50, 401–409 (2013).
Windpassinger, C. et al. Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nat. Genet. 36, 271–276 (2004).
Cui, X. et al. Seipin ablation in mice results in severe generalized lipodystrophy. Hum. Mol. Genet. 20, 3022–3030 (2011).
Chen, W. et al. Berardinelli-seip congenital lipodystrophy 2/seipin is a cell-autonomous regulator of lipolysis essential for adipocyte differentiation. Mol. Cell Biol. 32, 1099–1111 (2012).
Prieur, X. et al. Thiazolidinediones partially reverse the metabolic disturbances observed in Bscl2/seipin-deficient mice. Diabetologia 56, 1813–1825 (2013).
Chen, W., Zhou, H., Saha, P., Li, L. & Chan, L. Molecular mechanisms underlying fasting modulated liver insulin sensitivity and metabolism in male lipodystrophic Bscl2/Seipin-deficient mice. Endocrinology 155, 4215–4225 (2014).
Liu, L. et al. Adipose-specific knockout of Seipin/Bscl2 results in progressive lipodystrophy. Diabetes 63, 2320–2331 (2014).
Ebihara, C. et al. Seipin is necessary for normal brain development and spermatogenesis in addition to adipogenesis. Hum. Mol. Genet. 24, 4238–4249 (2015).
Chen, W. et al. Altered lipid metabolism in residual white adipose tissues of Bscl2 deficient mice. PLoS ONE 8, e82526 (2013).
Boutet, E. et al. Seipin deficiency alters fatty acid Δ9 desaturation and lipid droplet formation in Berardinelli-Seip congenital lipodystrophy. Biochimie 91, 796–803 (2009).
Online Mendelian Inheritance in Man©. #612526 Lipodystrophy, congenital generalized, type 3; CGL3 [online], (2014).
Engelman, J. A., Zhang, X. L., Galbiati, F. & Lisanti, M. P. Chromosomal localization, genomic organization, and developmental expression of the murine caveolin gene family (Cav-1, -2, and -3). Cav-1 and Cav-2 genes map to a known tumor suppressor locus (6-A2/7q31). FEBS Lett. 429, 330–336 (1998).
Stan, R. V. Structure of caveolae. Biochim. Biophys. Acta 1746, 334–348 (2005).
Parton, R. G. & Simons, K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 8, 185–194 (2007).
Razani, B. & Lisanti, M. P. Caveolin-deficient mice: insights into caveolar function human disease. J. Clin. Invest. 108, 1553–1561 (2001).
Blouin, C. M. et al. Lipid droplet analysis in caveolin-deficient adipocytes: alterations in surface phospholipid composition and maturation defects. J. Lipid Res. 51, 945–956 (2010).
Briand, N. et al. Caveolin-1 expression and cavin stability regulate caveolae dynamics in adipocyte lipid store fluctuation. Diabetes 63, 4032–4044 (2014).
Bosch, M. et al. Caveolin-1 deficiency causes cholesterol-dependent mitochondrial dysfunction and apoptotic susceptibility. Curr. Biol. 21, 681–686 (2011).
Asterholm, I. W., Mundy, D. I., Weng, J., Anderson, R. G. & Scherer, P. E. Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metab. 15, 171–185 (2012).
Williams, T. M. & Lisanti, M. P. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am. J. Physiol. Cell Physiol. 288, C494–C506 (2005).
Le Lay, S. & Kurzchalia, T. V. Getting rid of caveolins: phenotypes of caveolin-deficient animals. Biochim. Biophys. Acta 1746, 322–333 (2005).
Drab, M. et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293, 2449–2452 (2001).
Austin, E. D. et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ. Cardiovasc. Genet. 5, 336–343 (2012).
Online Mendelian Inheritance in Man©. #613327 Lipodystrophy, congenital generalized, type 4; CGL4 [online], (2013).
Liu, L. & Pilch, P. F. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J. Biol. Chem. 283, 4314–4322 (2008).
Perez-Diaz, S. et al. Polymerase I and transcript release factor (PTRF) regulates adipocyte differentiation and determines adipose tissue expandability. FASEB J. 28, 3769–3779 (2014).
Rajab, A., Heathcoate, K., Joshi, S., Jeffery, S. & Patton, M. Heterogeneity for congenital generalized lipodystrophy in seventeen patients from Oman. Am. J. Hum. Genet. 110, 219–225 (2002).
Simha, V., Agarwal, A. K., Aronin, P. A., Iannaccone, S. T. & Garg, A. Novel subtype of congenital generalized lipodystrophy associated with muscular weakness and cervical spine instability. Am. J. Med. Genet. A 146A, 2318–2326 (2008).
Liu, L. et al. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 8, 310–317 (2008).
Ding, S. Y. et al. Pleiotropic effects of cavin-1 deficiency on lipid metabolism. J. Biol. Chem. 289, 8473–8483 (2014).
GeneTests [online], (2015).
Cortes, V. A. et al. Divergent metabolic phenotype between two sisters with congenital generalized lipodystrophy due to double AGPAT2 homozygous mutations. A clinical, genetic and in silico study. PLoS ONE 9, e87173 (2014).
Agarwal, A. K. & Garg, A. Genetic basis of lipodystrophies and management of metabolic complications. Annu. Rev. Med. 57, 297–311 (2006).
Agarwal, A. K. et al. Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: biochemical characterization and inability to rescue hepatic steatosis in Agpat2−/− gene lipodystrophic mice. J. Biol. Chem. 286, 37676–37691 (2011).
Sankella, S., Garg, A., Horton, J. D. & Agarwal, A. K. Hepatic gluconeogenesis is enhanced by phosphatidic acid which remains uninhibited by insulin in lipodystrophic Agpat2−/− mice. J. Biol. Chem. 289, 4762–4777 (2014).
Petersen, K. F. et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Invest. 109, 1345–1350 (2002).
Muniyappa, R. et al. Effects of leptin replacement therapy on pancreatic β-cell function in patients with lipodystrophy. Diabetes Care 37, 1101–1107 (2014).
Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 18, 139–143 (2011).
Okada, E., Iwahira, Y. & Maruyama, Y. Buttock deformity repair for congenital generalized lipodystrophy. Plast. Reconstr. Surg. 95, 744–746 (1995).
[No authors listed]. Metreleptin (Myalept): a leptin analog for generalized lipodystrophy. Med. Lett. Drugs Ther. 57, 13–14 (2015).
Simha, V. & Garg, A. Inherited lipodystrophies and hypertriglyceridemia. Curr. Opin. Lipidol. 20, 300–308 (2009).
Handelsman, Y. et al. The clinical approach to the detection of lipodystrophy - an AACE consensus statement. Endocr. Pract. 19, 107–116 (2013).
Napolitano, C. & Priori, S. G. Diagnosis and treatment of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 4, 675–678 (2007).
Prieur, X., Le May, C., Magre, J. & Cariou, B. Congenital lipodystrophies and dyslipidemias. Curr. Atheroscler. Rep. 16, 437 (2014).
Araujo-Vilar, D. et al. Recombinant human leptin treatment in genetic lipodystrophic syndromes: the long-term Spanish experience. Endocrine 49, 139–147 (2014).
Beltrand, J. et al. Metabolic correction induced by leptin replacement treatment in young children with Berardinelli-Seip congenital lipoatrophy. Pediatrics 120, e291–e296 (2007).
Chan, J. L. et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr. Pract. 17, 922–932 (2011).
Safar Zadeh, E. et al. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J. Hepatol. 59, 131–137 (2013).
Ebihara, K. et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J. Clin. Endocrinol. Metab. 92, 532–541 (2007).
FDA. Endocrinologic and metabolic drugs advisory committee briefing document: METRELEPTIN (BLA STN125390) [online], (2013).
Oral, E. A. & Chan, J. L. Rationale for leptin-replacement therapy for severe lipodystrophy. Endocr. Pract. 16, 324–333 (2010).
Cortes, V. A. et al. Leptin ameliorates insulin resistance and hepatic steatosis in Agpat2−/− lipodystrophic mice independent of hepatocyte leptin receptors. J. Lipid Res. 55, 276–288 (2014).
Chou, K. & Perry, C. M. Metreleptin: first global approval. Drugs 73, 989–997 (2013).
Barter, P. J. et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357, 2109–2122 (2007).
Okamoto, H. et al. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature 406, 203–207 (2000).
Robciuc, M. R. et al. Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler. Thromb. Vasc. Biol. 33, 1706–1713 (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Neuschwander-Tetri, B. A. et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015).
Musso, C. et al. Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): a 30-year prospective. Medicine (Baltimore) 83, 209–222 (2004).
Pivnick, E. K. et al. Neonatal progeroid (Wiedemann-Rautenstrauch) syndrome: report of five new cases and review. Am. J. Med. Genet. 90, 131–140 (2000).
Hou, J. W. Natural course of neonatal progeroid syndrome. Pediatr. Neonatol. 50, 102–109 (2009).
Acknowledgements
A.G. acknowledges grant support from the NIH RO1 DK105448, CTSA Grant UL1 RR024982 and Southwest Medical Foundation. We thank P.-Y. Tseng for help with illustrations and mutational screening.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
A.G. co-holds a patent regarding the use of leptin for treating human lipoatrophy and the method of determining predisposition to this treatment but receives no financial compensation. A.G. has received research grants from Aegerion, Astra-Zeneca, Bristol-Myers-Squibb and Pfizer and is a consultant for Amgen, Back Bay Life Sciences, Biomarin Pharmaceuticals, BioMedical Insights, Clearview Healthcare, Eli Lilly, Engage Health, Gerson Lehrman Group, Health Advances, Ipsen Pharmaceuticals, Intellisphere, Medscape and Tekmira. A.G. is also an advisory board member for AstraZeneca. N.P. declares no competing interests.
Rights and permissions
About this article
Cite this article
Patni, N., Garg, A. Congenital generalized lipodystrophies—new insights into metabolic dysfunction. Nat Rev Endocrinol 11, 522–534 (2015). https://doi.org/10.1038/nrendo.2015.123
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2015.123
This article is cited by
-
Translocation of gut microbes to epididymal white adipose tissue drives lipid metabolism disorder under heat stress
Science China Life Sciences (2023)
-
Identifying congenital generalized lipodystrophy using deep learning-DEEPLIPO
Scientific Reports (2023)
-
The neonatal onset diabetes mellitus of Chinese neonate with congenital generalized lipodystrophy 2: a case report
BMC Endocrine Disorders (2022)
-
Type 2 congenital generalized lipodystrophy with a heterozygous missense NOTCH2 mutation
European Journal of Clinical Nutrition (2022)
-
Emerging Insights into the Molecular Architecture of Caveolin-1
The Journal of Membrane Biology (2022)