Key Points
-
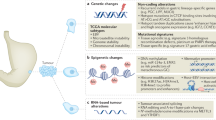
Globally, gastric cancer is the fourth most common cancer in men and fifth most common cancer in women
-
Helicobacter pylori is the most intriguing and best studied risk factor for sporadic gastric cancers
-
Signalling pathways and cancer stem cells have key roles in carcinogenesis and progression
-
Localized gastric cancer can be cured by primary surgery, but adjunctive therapies increase the cure rate
-
New somatic genes altered in gastric cancer (such as ARID1A, FAT4, MLL and KMT2C) have been identified, but thorough interrogation of gastric cancer is as yet incomplete
-
Therapeutic approaches that exploit the capabilities of the host immune mechanisms hold immense promise
Abstract
Gastric cancer imposes a considerable health burden around the globe despite its declining incidence. The disease is often diagnosed in advanced stages and is associated with a poor prognosis for patients. An in-depth understanding of the molecular underpinnings of gastric cancer has lagged behind many other cancers of similar incidence and morbidity, owing to our limited knowledge of germline susceptibility traits for risk and somatic drivers of progression (to identify novel therapeutic targets). A few germline (PLCE1) and somatic (ERBB2, ERBB3, PTEN, PI3K/AKT/mTOR, FGF, TP53, CDH1 and MET) alterations are emerging and some are being pursued clinically. Novel somatic gene targets (ARID1A, FAT4, MLL and KMT2C) have also been identified and are of interest. Variations in the therapeutic approaches dependent on geographical region are evident for localized gastric cancer—differences that are driven by preferences for the adjuvant strategies and the extent of surgery coupled with philosophical divides. However, greater uniformity in approach has been noted in the metastatic cancer setting, an incurable condition. Having realized only modest successes, momentum is building for carrying out more phase III comparative trials, with some using biomarker-based patient selection strategies. Overall, rapid progress in biotechnology is improving our molecular understanding and can help with new drug discovery. The future prospects are excellent for defining biomarker-based subsets of patients and application of specific therapeutics. However, many challenges remain to be tackled. Here, we review representative molecular and clinical dimensions of gastric cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jemal, A. et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011).
National Cancer Institute at the National Institutes of Health. Cellular Classification of Gastric Cancer [online], (2013).
Bertuccio, P. et al. Recent patterns in gastric cancer: a global overview. Int. J. Cancer 125, 666–673 (2009).
Chen, J., Bu, X. L., Wang, Q. Y., Hu, P. J. & Chen, M. H. Decreasing seroprevalence of Helicobacter pylori infection during 1993–2003 in Guangzhou, southern China. Helicobacter 12, 164–169 (2007).
Kawakami, E., Machado, R. S., Ogata, S. K. & Langner, M. Decrease in prevalence of Helicobacter pylori infection during a 10-year period in Brazilian children. Arq. Gastroenterol. 45, 147–151 (2008).
Tkachenko, M. A. et al. Dramatic changes in the prevalence of Helicobacter pylori infection during childhood: a 10-year follow-up study in Russia. J. Pediatr. Gastroenterol. Nutr. 45, 428–432 (2007).
Lee, K. J. et al. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. Int. J. Cancer 118, 2315–2321 (2006).
Parkin, D. M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 118, 3030–3044 (2006).
Axon, A. Review article: gastric cancer and Helicobacter pylori. Aliment. Pharmacol. Ther. 16 (Suppl. 4), 83–88 (2002).
Kim, S. S., Ruiz, V. E., Carroll, J. D. & Moss, S. F. Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett. 305, 228–238 (2011).
Conteduca, V. et al. H. pylori infection and gastric cancer: state of the art (review). Int. J. Oncol. 42, 5–18 (2013).
Rizzato, C. et al. Risk of advanced gastric precancerous lesions in Helicobacter pylori infected subjects is influenced by ABO blood group and cagA status. Int. J. Cancer 133, 315–322 (2013).
Correa, P. & Houghton, J. Carcinogenesis of Helicobacter pylori. Gastroenterology 133, 659–672 (2007).
Tsugawa, H. et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe 12, 764–777 (2012).
Pimentel-Nunes, P. et al. Helicobacter pylori induces increased expression of Toll-like receptors and decreased toll-interacting protein in gastric mucosa that persists throughout gastric carcinogenesis. Helicobacter 18, 22–32 (2013).
Peek, R. M. Jr et al. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J. Natl Cancer Inst. 89, 863–868 (1997).
Graham, D. Y. & Yamaoka, Y. H. pylori and cagA: relationships with gastric cancer, duodenal ulcer, and reflux esophagitis and its complications. Helicobacter 3, 145–151 (1998).
Hatakeyama, M. Helicobacter pylori cagA—a potential bacterial oncoprotein that functionally mimics the mammalian Gab family of adaptor proteins. Microbes Infect. 5, 143–150 (2003).
Mueller, D. et al. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J. Clin. Invest. 122, 1553–1566 (2012).
Müller, A. Multistep activation of the Helicobacter pylori effector CagA. J. Clin. Invest. 122, 1192–1195 (2012).
Higashi, H. et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295, 683–686 (2002).
Meyer-ter-Vehn, T., Covacci, A., Kist, M. & Pahl, H. L. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J. Biol. Chem. 275, 16064–16072 (2000).
Schirrmeister, W. et al. Ectodomain shedding of E-cadherin and c-Met is induced by Helicobacter pylori infection. Exp. Cell Res. 315, 3500–3508 (2009).
Murata-Kamiya, N. et al. Helicobacter pylori cagA interacts with E-cadherin and deregulates the β-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene 26, 4617–4626 (2007).
Suzuki, M. et al. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J. Exp. Med. 202, 1235–1247 (2005).
Hatakeyama, M. Linking epithelial polarity and carcinogenesis by multitasking Helicobacter pylori virulence factor cagA. Oncogene 27, 7047–7054 (2008).
Bartchewsky, W. Jr et al. Effect of Helicobacter pylori infection on IL-8, IL-1β and COX-2 expression in patients with chronic gastritis and gastric cancer. Scand. J. Gastroenterol. 44, 153–161 (2009).
Li, C. Q., Pignatelli, B. & Ohshima, H. Coexpression of interleukin-8 and inducible nitric oxide synthase in gastric mucosa infected with cagA+ Helicobacter pylori. Dig. Dis. Sci. 45, 55–62 (2000).
Suganuma, M. et al. TNF-α-inducing protein, a carcinogenic factor secreted from H. pylori, enters gastric cancer cells. Int. J. Cancer 123, 117–122 (2008).
Brandt, S., Kwok, T., Hartig, R., Konig, W. & Backert, S. NF-κB activation and potentiation of proinflammatory responses by the Helicobacter pylori cagA protein. Proc. Natl Acad. Sci. USA 102, 9300–9305 (2005).
Rad, R. et al. Synergistic effect of Helicobacter pylori virulence factors and interleukin-1 polymorphisms for the development of severe histological changes in the gastric mucosa. J. Infect. Dis. 188, 272–281 (2003).
Zambon, C. F. et al. Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine 29, 141–152 (2005).
Chang, Y. J. et al. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-κB activation. Mol. Pharmacol. 66, 1465–1477 (2004).
Walduck, A. K. et al. Identification of novel cyclooxygenase-2-dependent genes in Helicobacter pylori infection in vivo. Mol. Cancer 8, 22 (2009).
Seo, J. H., Kim, H. & Kim, K. H. Cyclooxygenase-2 expression by transcription factors in Helicobacter pylori-infected gastric epithelial cells: comparison between HP 99 and NCTC 11637. Ann. NY Acad. Sci. 973, 477–480 (2002).
Liu, A. Q., Ge, L. Y., Ye, X. Q., Luo, X. L. & Luo, Y. Reduced FAF1 expression and Helicobacter infection: correlations with clinicopathological features in gastric cancer. Gastroenterol. Res. Pract. 2012, 153219 (2012).
Wang, G. et al. Involvement of aquaporin 3 in Helicobacter pylori-related gastric diseases. PLoS ONE 7, e49104 (2012).
Tamura, G. et al. E-cadherin gene promoter hypermethylation in primary human gastric carcinomas. J. Natl Cancer Inst. 92, 569–573 (2000).
Becker, K. F. & Höfler, H. Frequent somatic allelic inactivation of the E-cadherin gene in gastric carcinomas. J. Natl Cancer Inst. 87, 1082–1084 (1995).
Peterson, A. J. et al. Helicobacter pylori infection promotes methylation and silencing of trefoil factor 2, leading to gastric tumor development in mice and humans. Gastroenterology 139, 2005–2017 (2010).
Katayama, Y., Takahashi, M. & Kuwayama, H. Helicobacter pylori causes RUNX3 gene methylation and its loss of expression in gastric epithelial cells, which is mediated by nitric oxide produced by macrophages. Biochem. Biophys. Res. Commun. 388, 496–500 (2009).
Yoshida, T. et al. Altered mucosal DNA methylation in parallel with highly active Helicobacter pylori-related gastritis. Gastric Cancer http://dx.doi.org/10.1007/s10120-012-0230-x.
Matsumoto, Y. et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat. Med. 13, 470–476 (2007).
Holcombe, C. Helicobacter pylori: the African enigma. Gut 33, 429–431 (1992).
Guilford, P. et al. E-cadherin germline mutations in familial gastric cancer. Nature 392, 402–405 (1998).
Huntsman, D. G. et al. Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. N. Engl. J. Med. 344, 1904–1909 (2001).
Hu, Z., Ajani, J. A. & Wei, Q. Molecular epidemiology of gastric cancer: current status and future prospects. Gastrointest. Cancer Res. 1, 12–19 (2007).
Wang, L. D. et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat. Genet. 42, 759–763 (2010).
Study Group of Millennium Genome Project for Cancer. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat. Genet. 40, 730–740 (2008).
Wang, T., Zhang, L., Li, H., Wang, B. & Chen, K. Prostate stem cell antigen polymorphisms and susceptibility to gastric cancer: a systematic review and meta-analysis. Cancer Epidemiol. Biomarkers Prev. 21, 843–850 (2012).
Saeki, N., Gu, J., Yoshida, T. & Wu, X. Prostate stem cell antigen: a Jekyll and Hyde molecule? Clin. Cancer Res. 16, 3533–3538 (2010).
Abnet, C. C. et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat. Genet. 42, 764–767 (2010).
Zhang, H. et al. Genetic variants at 1q22 and 10q23 reproducibly associated with gastric cancer susceptibility in a Chinese population. Carcinogenesis 32, 848–852 (2011).
Wang, M. et al. Potentially functional variants of PLCE1 identified by GWASs contribute to gastric adenocarcinoma susceptibility in an eastern Chinese population. PLoS ONE 7, e31932 (2012).
Luo, D. et al. Genetic variation in PLCE1 is associated with gastric cancer survival in a Chinese population. J. Gastroenterol. 46, 1260–1266 (2011).
Palmer, A. J. et al. Genetic variation in C20orf54, PLCE1 and MUC1 and the risk of upper gastrointestinal cancers in Caucasian populations. Eur. J. Cancer Prev. 21, 541–544 (2012).
Shi, Y. et al. A genome-wide association study identifies new susceptibility loci for non-cardia gastric cancer at 3q13.31 and 5p13.1. Nat. Genet. 43, 1215–1218 (2011).
Wu, K. C. et al. Molecular basis of therapeutic approaches to gastric cancer. J. Gastroenterol. Hepatol. 24, 37–41 (2009).
Wong, H. & Yau, T. Targeted therapy in the management of advanced gastric cancer: are we making progress in the era of personalized medicine? Oncologist 17, 346–358 (2012).
Schneider, B. G. et al. Promoter DNA hypermethylation in gastric biopsies from subjects at high and low risk for gastric cancer. Int. J. Cancer 127, 2588–2597 (2010).
Markman, B., Dienstmann, R. & Tabernero, J. Targeting the PI3K/Akt/mTOR pathway—beyond rapalogs. Oncotarget 1, 530–543 (2010).
Chalhoub, N. & Baker, S. J. PTEN and the PI3-kinase pathway in cancer. Annu. Rev. Pathol. 4, 127–150 (2009).
Bergfeld, S. A. & DeClerck, Y. A. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev. 29, 249–261 (2010).
Takaishi, S., Okumura, T. & Wang, T. C. Gastric cancer stem cells. J. Clin. Oncol. 26, 2876–2882 (2008).
Chen, C. N. et al. Gene expression profile predicts patient survival of gastric cancer after surgical resection. J. Clin. Oncol. 23, 7286–7295 (2005).
Cho, J. Y. et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin. Cancer Res. 17, 1850–1857 (2011).
Deng, N. et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 61, 673–684 (2012).
Ivanova, T. et al. Integrated epigenomics identifies BMP4 as a modulator of cisplatin sensitivity in gastric cancer. Gut 62, 22–33 (2013).
Kim, H. K. et al. A gene expression signature of acquired chemoresistance to cisplatin and fluorouracil combination chemotherapy in gastric cancer patients. PLoS ONE 6, e16694 (2011).
Kim, H. K. et al. Three-gene predictor of clinical outcome for gastric cancer patients treated with chemotherapy. Pharmacogenomics J. 12, 119–127 (2012).
Ooi, C. H. et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 5, e1000676 (2009).
Takeno, A. et al. Gene expression profile prospectively predicts peritoneal relapse after curative surgery of gastric cancer. Ann. Surg. Oncol. 17, 1033–1042 (2010).
Tan, I. B. et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology 141, 476–485 (2011).
Wu, Y. et al. Comprehensive genomic meta-analysis identifies intra-tumoural stroma as a predictor of survival in patients with gastric cancer. Gut 26, 1100–1111 (2013).
Zang, Z. J. et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat. Genet. 44, 570–574 (2012).
Zouridis, H. et al. Methylation subtypes and large-scale epigenetic alterations in gastric cancer. Sci. Transl. Med. 4, 156ra140 (2012).
Tan, D. S., Gerlinger, M., Teh, B. T. & Swanton, C. Anti-cancer drug resistance: understanding the mechanisms through the use of integrative genomics and functional RNA interference. Eur. J. Cancer 46, 2166–2177 (2010).
Subramaniam, D., Ramalingam, S., Houchen, C. W. & Anant, S. Cancer stem cells: a novel paradigm for cancer prevention and treatment. Mini Rev. Med. Chem. 10, 359–371 (2010).
Mani, S. A. et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Peacock, C. D. & Watkins, D. N. Cancer stem cells and the ontogeny of lung cancer. J. Clin. Oncol. 26, 2883–2889 (2008).
Peinado, H., Olmeda, D. & Cano, A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer 7, 415–428 (2007).
Voutsadakis, I. A. The ubiquitin-proteasome system and signal transduction pathways regulating epithelial mesenchymal transition of cancer. J. Biomed. Sci. 19, 67 (2012).
Li, Y. Q. et al. c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proc. Natl Acad. Sci. USA 108, 9951–9956 (2011).
Roberts, A. B. & Wakefield, L. M. The two faces of transforming growth factor β in carcinogenesis. Proc. Natl Acad. Sci. USA 100, 8621–8623 (2003).
Bierie, B. & Moses, H. L. TGFβ: the molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 6, 506–520 (2006).
Watabe, T. & Miyazono, K. Roles of TGF-β family signaling in stem cell renewal and differentiation. Cell Res. 19, 103–115 (2009).
Singh, A. & Settleman, J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29, 4741–4751 (2010).
Zhou, J. B. et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl Acad. Sci. USA 104, 16158–16163 (2007).
Hirsch, H. A., Iliopoulos, D., Tsichlis, P. N. & Struhl, K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 69, 7507–7511 (2009).
Singh, B. N., Fu, J., Srivastava, R. K. & Shankar, S. Hedgehog signaling antagonist GDC-0449 (vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms. PLoS ONE 6, e27306 (2011).
Heindl, S. et al. Relevance of MET activation and genetic alterations of KRAS and E-cadherin for cetuximab sensitivity of gastric cancer cell lines. J. Cancer Res. Clin. Oncol. 138, 843–858 (2012).
Komuro, A. et al. Diffuse-type gastric carcinoma: progression, angiogenesis, and transforming growth factor β signaling. J. Natl Cancer Inst. 101, 592–604 (2009).
Ebi, M., Kataoka, H., Higashiyama, S. & Joh, T. TGFβ induces EGFR transactivation and HB-EGF C-terminal fragment nuclear translocation through ADAM17 activation in gastric cancer cells. Gastroenterology 140 (Suppl. 1), S629 (2011).
Kang, M. H. et al. Inhibition of PI3 kinase/Akt pathway is required for BMP2-induced EMT and invasion. Oncol. Rep. 22, 525–534 (2009).
Takahashi, Y. et al. Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clin. Cancer Res. 2, 1679–1684 (1996).
Sharma, V. K., Vasudeva, R. & Howden, C. W. Changing demographics of gastric cancer: 15 year experience. Gastroenterology 114 (Suppl. 1), A40 (1998).
Takaishi, S. et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 27, 1006–1020 (2009).
Ishimoto, T. et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 19, 387–400 (2011).
Fornaro, L. et al. Anti-HER agents in gastric cancer: from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 8, 369–383 (2011).
Schlessinger, J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science 306, 1506–1507 (2004).
Dhanasekaran, D. N. & Johnson, G. L. MAPKs: function, regulation, role in cancer and therapeutic targeting. Oncogene 26, 3097–3099 (2007).
Lordick, F. et al. HER2 status of advanced gastric cancer is similar in Europe and Asia [abstract 253]. Ann. Oncol. 18 (Suppl. 7), vii95–vii96 (2007).
Zhang, X. L. et al. Comparative study on overexpression of HER2/neu and HER3 in gastric cancer. World J. Surg. 33, 2112–2118 (2009).
Terashima, M. et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin. Cancer Res. 18, 5992–6000 (2012).
Browne, B. C. et al. Inhibition of IGF1R activity enhances response to trastuzumab in HER-2-positive breast cancer cells. Ann. Oncol. 22, 68–73 (2011).
Wilkinson, N. W. et al. Epidermal growth factor receptor expression correlates with histologic grade in resected esophageal adenocarcinoma. J. Gastrointest. Surg. 8, 448–453 (2004).
Isinger-Ekstrand, A. et al. Genetic profiles of gastroesophageal cancer: combined analysis using expression array and tiling array-comparative genomic hybridization. Cancer Genet. Cytogenet. 200, 120–126 (2010).
Moutinho, C. et al. Epidermal growth factor receptor structural alterations in gastric cancer. BMC Cancer 8, 10 (2008).
Liu, Z. M. et al. Epidermal growth factor receptor mutation in gastric cancer. Pathology 43, 234–238 (2011).
Pinto, C. et al. Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study). Br. J. Cancer 101, 1261–1268 (2009).
Jaiswal, B. S. et al. Oncogenic ERBB3 mutations in human cancers. Cancer Cell 23, 603–617 (2013).
Migliore, C. & Giordano, S. Molecular cancer therapy: can our expectation be MET? Eur. J. Cancer 44, 641–651 (2008).
El-Rifai, W. & Powell, S. M. Molecular biology of gastric cancer. Semin. Radiat. Oncol. 12, 128–140 (2002).
Smolen, G. A. et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc. Natl Acad. Sci. USA 103, 2316–2321 (2006).
Janjigian, Y. Y. et al. MET expression and amplification in patients with localized gastric cancer. Cancer Epidemiol. Biomarkers Prev. 20, 1021–1027 (2011).
Lee, J. et al. Impact of MET amplification on gastric cancer: possible roles as a novel prognostic marker and a potential therapeutic target. Oncol. Rep. 25, 1517–1524 (2011).
Guo, A. et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc. Natl Acad. Sci. USA 105, 692–697 (2008).
Arteaga, C. L. HER3 and mutant EGFR meet MET. Nat. Med. 13, 675–677 (2007).
Corso, S., Comoglio, P. M. & Giordano, S. Cancer therapy: can the challenge be MET? Trends Mol. Med. 11, 284–292 (2005).
Corso, S. et al. Activation of HER family members in gastric carcinoma cells mediates resistance to MET inhibition. Mol. Cancer 9, 121 (2010).
Bachleitner-Hofmann, T. et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol. Cancer Ther. 7, 3499–3508 (2008).
Byun, D. S. et al. Frequent monoallelic deletion of PTEN and its reciprocal association with PIK3CA amplification in gastric carcinoma. Int. J. Cancer 104, 318–327 (2003).
Oki, E. et al. Impact of PTEN/AKT/PI3K signal pathway on the chemotherapy for gastric cancer [abstract 4034]. J. Clin. Oncol. 24 (Suppl. 18), 187s (2006).
Garrett, J. T. et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc. Natl Acad. Sci. USA 108, 5021–5026 (2011).
Shin, E. Y. et al. Up-regulation and co-expression of fibroblast growth factor receptors in human gastric cancer. J. Cancer Res. Clin. Oncol. 126, 519–528 (2000).
Wang, K. et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat. Genet. 43, 1219–1223 (2011).
Song, S. & Ajani, J. A. The role of microRNAs in cancers of the upper gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 10, 109–118 (2013).
Li, T. et al. MicroRNA-296-5p increases proliferation in gastric cancer through repression of Caudal-related homeobox 1. Oncogene http://dx.doi.org/10.1038/onc.2012.637.
Wang, M. et al. Overexpressed miR-301a promotes cell proliferation and invasion by targeting RUNX3 in gastric cancer. J. Gastroenterol. http://dx.doi.org/10.1007/s00535-012-0733–6.
Wang, M. et al. miR-17-5p/20a are important markers for gastric cancer and murine double minute 2 participates in their functional regulation. Eur. J. Cancer 49, 2010–2021 (2013).
Wu, X. et al. MicroRNA expression signatures during malignant progression from Barrett's esophagus to esophageal adenocarcinoma. Cancer Prev. Res. (Phila.) 6, 196–205 (2013).
Zhou, C. et al. microRNA-372 maintains oncogene characteristics by targeting TNFAIP1 and affects NFκB signaling in human gastric carcinoma cells. Int. J. Oncol. 42, 635–642 (2013).
Zhi, Q. et al. Oncogenic miR-544 is an important molecular target in gastric cancer. Anticancer Agents Med. Chem. 13, 270–275 (2013).
Wang, Y. Y. et al. Clinicopathologic significance of miR-10b expression in gastric carcinoma. Hum. Pathol. 44, 1278–1285 (2013).
Deng, H. et al. MicroRNA-195 and microRNA-378 mediate tumor growth suppression by epigenetical regulation in gastric cancer. Gene 518, 351–359 (2013).
Wen, D. et al. miR-133b acts as a tumor suppressor and negatively regulates FGFR1 in gastric cancer. Tumour Biol. 34, 793–803 (2013).
Saito, Y. et al. The tumor suppressor microRNA-29c is downregulated and restored by celecoxib in human gastric cancer cells. Int. J. Cancer 132, 1751–1760 (2012).
Cao, W. et al. Expression and regulatory function of miRNA-34a in targeting survivin in gastric cancer cells. Tumour Biol. 34, 963–971 (2012).
Zheng, L. et al. miRNA-145 targets v-ets erythroblastosis virus E26 oncogene homolog 1 to suppress the invasion, metastasis, and angiogenesis of gastric cancer cells. Mol. Cancer Res. 11, 182–193 (2013).
Liu, K. et al. Decreased expression of microRNA let-7i and its association with chemotherapeutic response in human gastric cancer. World J. Surg. Oncol. 10, 225 (2012).
Akiyoshi, S. et al. Clinical significance of miR-144-ZFX axis in disseminated tumour cells in bone marrow in gastric cancer cases. Br. J. Cancer 107, 1345–1353 (2012).
He, X. P. et al. Downregulation of miR-101 in gastric cancer correlates with cyclooxygenase-2 overexpression and tumor growth. FEBS J. 279, 4201–4212 (2012).
Crone, S. G. et al. microRNA-146a inhibits G. protein-coupled receptor-mediated activation of NF-κB by targeting CARD10 and COPS8 in gastric cancer. Mol. Cancer 11, 71 (2012).
Ichikawa, D., Komatsu, S., Konishi, H. & Otsuji, E. Circulating microRNA in digestive tract cancers. Gastroenterology 142, 1074–1078.e1 (2012).
Cui, L. et al. Gastric juice microRNAs as potential biomarkers for the screening of gastric cancer. Cancer 119, 1618–1626 (2013).
Zhang, X. et al. Gastric juice microRNA-421 is a new biomarker for screening gastric cancer. Tumour Biol. 33, 2349–2355 (2012).
Cai, H. et al. Plasma microRNAs serve as novel potential biomarkers for early detection of gastric cancer. Med. Oncol. 30, 452 (2013).
Li, C. et al. MiRNA-199a-3p in plasma as a potential diagnostic biomarker for gastric cancer. Ann. Surg. Oncol. http://dx.doi.org/10.1245/s10434-012-2600–3.
Wang, M. et al. Circulating miR-17-5p and miR-20a: molecular markers for gastric cancer. Mol. Med. Report 5, 1514–1520 (2012).
Valladares-Ayerbes, M. et al. Circulating miR-200c as a diagnostic and prognostic biomarker for gastric cancer. J. Transl. Med. 10, 186 (2012).
Stern, H. M. Improving treatment of HER2-positive cancers: opportunities and challenges. Sci. Transl. Med. 4, 127rv2 (2012).
Nahta, R. Molecular mechanisms of trastuzumab-based treatment in HER2-overexpressing breast cancer. ISRN Oncol. 2012, 428062 (2012).
Kim, J. W. et al. The growth inhibitory effect of lapatinib, a dual inhibitor of EGFR and HER2 tyrosine kinase, in gastric cancer cell lines. Cancer Lett. 272, 296–306 (2008).
Lu, Y., Zi, X. & Pollak, M. Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. Int. J. Cancer 108, 334–341 (2004).
Chen, C. T. et al. MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells. Mol. Cancer Ther. 11, 660–669 (2012).
Shattuck, D. L., Miller, J. K., Carraway, K. L. & Sweeney, C. Met receptor contributes to trastuzumab resistance of HER2-overexpressing breast cancer cells. Cancer Res. 68, 1471–1477 (2008).
Molina, M. A. et al. Trastuzumab (herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and activated HER2 ectodomain cleavage in breast cancer cells. Cancer Res. 61, 4744–4749 (2001).
Nagy, P. et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-Expressing breast cancer cell line. Cancer Res. 65, 473–482 (2005).
Gong, S. J., Jin, C. J., Rha, S. Y. & Chung, H. C. Growth inhibitory effects of trastuzumab and chemotherapeutic drugs in gastric cancer cell lines. Cancer Lett. 214, 215–224 (2004).
Arcaro, A. & Guerreiro, A. S. The phosphoinositide 3-kinase pathway in human cancer: genetic alterations and therapeutic implications. Curr. Genomics 8, 271–306 (2007).
Engelman, J. A. et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat. Med. 14, 1351–1356 (2008).
Eichhorn, P. J. et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 68, 9221–9230 (2008).
Korkaya, H. et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol. Cell 47, 570–584 (2012).
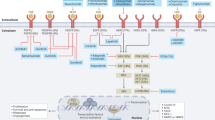
Shah, M. A. & Ajani, J. A. Gastric cancer—an enigmatic and heterogeneous disease. JAMA 303, 1753–1754 (2010).
Bickenbach, K. & Strong, V. E. Comparisons of gastric cancer treatments: East vs West. J. Gastric Cancer 12, 55–62 (2012).
Tamura, S., Takeno, A. & Miki, H. Lymph node dissection in curative gastrectomy for advanced gastric cancer. Int. J. Surg. Oncol. 2011, 748745 (2011).
Macdonald, J. S. et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N. Engl. J. Med. 345, 725–730 (2001).
Sakuramoto, S. et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 357, 1810–1820 (2007).
Bang, Y. J. et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 379, 315–321 (2012).
Ajani, J. A. et al. Gastric cancer. J. Natl Compr. Canc. Netw. 8, 378–409 (2010).
Lim, L., Michael, M., Mann, G. B. & Leong, T. Adjuvant therapy in gastric cancer. J. Clin. Oncol. 23, 6220–6232 (2005).
Ychou, M. et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 29, 1715–1721 (2011).
Paoletti, X. et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA 303, 1729–1737 (2010).
Cunningham, D. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 355, 11–20 (2006).
Smalley, S. R. et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J. Clin. Oncol. 30, 2327–2333 (2012).
Fuchs, C. S. et al. Postoperative adjuvant chemoradiation for gastric or gastroesophageal junction (GEJ) adenocarcinoma using epirubicin, cisplatin, and infusional (CI) 5-FU (ECF) before and after CI 5-FU and radiotherapy (CRT) compared with bolus 5-FU/LV before and after CRT: Intergroup trial CALGB 80101 [abstract 4003]. J. Clin. Oncol. 29 (Suppl.), 4003 (2011).
Lee, J. et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J. Clin. Oncol. 30, 268–273 (2012).
Sasako, M. et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 29, 4387–4393 (2011).
Dikken, J. L. et al. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer 11, 329 (2011).
Van Cutsem, E. et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J. Clin. Oncol. 24, 4991–4997 (2006).
Koizumi, W. et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 9, 215–221 (2008).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
FDA. Trastuzumab. Office of Medical Products and Tobacco [online], (2010).
Lordick, F. et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 14, 490–499 (2013).
Waddell, T. et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 14, 481–489 (2013).
Ohtsu, A. et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J. Clin. Oncol. 29, 3968–3976 (2011).
Van Cutsem, E. et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J. Clin. Oncol. 30, 2119–2127 (2012).
Ajani, J. A. et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J. Clin. Oncol. 28, 1547–1553 (2010).
Thuss-Patience, P. C. et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur. J. Cancer 47, 2306–2314 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Everolimus for advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J. Clin. Oncol. (in press).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Fuchs, C. S. et al. REGARD: a phase III, randomized, double-blind trial of ramucirumab and best supportive care (BSC) versus placebo and BSC in the treatment of metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma following disease progression on first-line platinum- and/or fluoropyrimidine-containing combination therapy [abstract LBA5]. J. Clin. Oncol. 30 (Suppl. 34), LBA5 (2012).
Ford, H. et al. Cougar-02: a randomized phase III study of docetaxel versus active symptom control in advanced esophagogastric adenocarcinoma [abstract LBA4]. J. Clin. Oncol. 30 (Suppl. 34), LBA4 (2012).
Bang, Y.-J. et al. A randomized, open-label, phase III study of lapatinib in combination with weekly paclitaxel versus weekly paclitaxel alone in the second-line treatment of HER2 amplified advanced gastric cancer (AGC) in Asian population: Tytan study [abstract 11]. J. Clin. Oncol. 30 (Suppl. 34), 11 (2012).
Lennerz, J. K. et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J. Clin. Oncol. 29, 4803–4810 (2011).
Oliner, K. S. et al. Evaluation of MET pathway biomarkers in a phase II study of rilotumumab (R, AMG 102) or placebo (P) in combination with epirubicin, cisplatin, and capecitabine (ECX) in patients (pts) with locally advanced or metastatic gastric (G) or esophagogastric junction (EGJ) cancer [abstract 4005]. J. Clin. Oncol. 30 (Suppl.), 4005 (2012).
Hwang, J. Y. et al. Recapitulation of previous genome-wide association studies with two distinct pathophysiological entities of gastric cancer in the Korean population. J. Hum. Genet. 58, 233–235 (2013).
Saeki, N. et al. A functional single nucleotide polymorphism in mucin 1, at chromosome 1q22, determines susceptibility to diffuse-type gastric cancer. Gastroenterology 140, 892–902 (2011).
Gu, H. et al. Replication study of PLCE1 and C20orf54 polymorphism and risk of esophageal cancer in a Chinese population. Mol. Biol. Rep. 39, 9105–9111 (2012).
Mammano, E. et al. Epidermal growth factor receptor (EGFR): mutational and protein expression analysis in gastric cancer. Anticancer Res. 26, 3547–3550 (2006).
Hong, S. P. et al. Overexpression of Notch 1 signaling associates with the tumorigenesis of gastric adenorna and intestinal type of gastric cancer. Gastroenterology 132, A617 (2007).
Yu, G. Z. et al. Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of Chinese patients with gastric cancer. Clin. Cancer Res. 15, 1821–1829 (2009).
Lang, S. A. et al. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int. J. Cancer 120, 1803–1810 (2007).
Zhou, Y. N. et al. Clinicopathological significance of E-cadherin, VEGF, and MMPs in gastric cancer. Tumor Biol. 31, 549–558 (2010).
Kitoh, T. et al. Increased expression of matrix metalloproteinase-7 in invasive early gastric cancer. J. Gastroenterol. 39, 434–440 (2004).
Fukaya, M. et al. Hedgehog signal activation in gastric pit cell and in diffuse-type gastric cancer. Gastroenterology 131, 14–29 (2006).
Hattori, Y. et al. Immunohistochemical detection of K-sam protein in stomach cancer. Clin. Cancer Res. 2, 1373–1381 (1996).
Pereira, P. S. et al. E-cadherin missense mutations, associated with hereditary diffuse gastric cancer (HDGC) syndrome, display distinct invasive behaviors and genetic interactions with the Wnt and Notch pathways in Drosophila epithelia. Hum. Mol. Genet. 15, 1704–1712 (2006).
Kobayashi, M., Kawashima, A., Mai, M. & Ooi, A. Analysis of chromosome 17p13 (p53 locus) alterations in gastric carcinoma cells by dual-color fluorescence in situ hybridization. Am. J. Pathol. 149, 1575–1584 (1996).
Guo, C. Y., Xu, X. F., Wu, L. Y. & Liu, S. F. PCR-SSCP-DNA sequencing method in detecting PTEN gene mutation and its significance in human gastric cancer. World J. Gastroenterol. 14, 3804–3811 (2008).
Li, H. et al. Insulin-like growth factor-I receptor blockade reduces tumor angiogenesis and enhances the effects of bevacizumab for a human gastric cancer cell line, MKN45. Cancer 117, 3135–3147 (2011).
Cepero, V. et al. MET and KRAS gene amplification mediates acquired resistance to MET tyrosine kinase inhibitors. Cancer Res. 70, 7580–7590 (2010).
Fuchs, C. et al. Postoperative adjuvant chemoradiation for gastric or gastroesophageal adenocarcinoma using epirubicin, cisplatin, and infusional (CI) 5-FU (ECF) before and after CI 5-FU and radiotherapy (RT): Interim toxicity results from Intergroup trial CALGB 80101 [abtract 4003]. J. Clin. Oncol. 29 (Suppl.), 4003 (2011).
Tsuburaya, A. et al. SAMIT: Preliminary safety data from a 2 × 2 factorial randomized phase III trial to investigate weekly paclitaxel (PTX) followed by oral fluoropyrimidines (FPs) versus FPs alone as adjuvant chemotherapy in patients (pts) with gastric cancer [abstract 4017]. J. Clin. Oncol. 29 (Suppl.), 4017 (2011).
Leong, T. L. et al. TOPGEAR: An international randomized phase III trial of preoperative chemoradiotherapy versus preoperative chemotherapy for resectable gastric cancer (AGITG/TROG/EORTC/NCIC CTG) [abstract TPS4141]. J. Clin. Oncol. 30 (Suppl.), TPS4141 (2012).
Lordick, F. et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 14, 490–499 (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Waddell, T. S. et al. A randomized, multicenter trial of epirubicin, oxaliplatin, and capecitabine (EOC) plus panitumumab in advanced esophagogastric cancer (REAL3) [abstract LBA4000]. J. Clin. Oncol. 30 (Suppl.), LBA4000 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Kang, J. H. et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J. Clin. Oncol. 30, 1513–1518 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2009).
Acknowledgements
The authors are supported by grants from the Caporella, Cantu, Smith, Park, Fairman, Frazier, Sultan, Oaks, Vansteklenberg and Milrod families, by the Kevin Fund, Schecter Private Fund and the Rivercreek Foundation and a Multidisciplinary Research Grant from University of Texas MD Anderson Cancer Center. J. A. Ajani is also supported by grants RO1CA138671 and ROaCA172741 awarded by the National Cancer Institute, Bethesda, MD, USA. The authors also thank the editorial staff of Nature Reviews Clinical Oncology for their editorial assistance in considerably improving the clarity and organization of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors researched the data, discussed the article's content, wrote the manuscript and edited it before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wadhwa, R., Song, S., Lee, JS. et al. Gastric cancer—molecular and clinical dimensions. Nat Rev Clin Oncol 10, 643–655 (2013). https://doi.org/10.1038/nrclinonc.2013.170
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2013.170
This article is cited by
-
Early prediction of response to palliative chemotherapy in patients with stage-IV gastric and esophageal cancer
BMC Cancer (2023)
-
Clinicopathological and prognostic significance of LKB1 expression in gastric cancer: a systematic review and meta-analysis
Scientific Reports (2023)
-
Tripartite motif 31 drives gastric cancer cell proliferation and invasion through activating the Wnt/β-catenin pathway by regulating Axin1 protein stability
Scientific Reports (2023)
-
Novel evidence for m6A methylation regulators as prognostic biomarkers and FTO as a potential therapeutic target in gastric cancer
British Journal of Cancer (2022)
-
CCT5 induces epithelial-mesenchymal transition to promote gastric cancer lymph node metastasis by activating the Wnt/β-catenin signalling pathway
British Journal of Cancer (2022)