Key Points
-
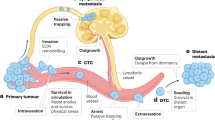
Metastasis is the spread of cancer from its site of origin and subsequent colonization of distant organs. Until recently, studying the details of this process has been difficult owing to the limited number of cells involved and the inaccessibility of the relevant anatomical sites.
-
Optical imaging enables the process of metastasis to be observed and studied directly. Resolution can vary from the level of whole tissues down to sub-cellular structures depending on the imaging method.
-
In addition to detecting tumour cells, imaging can provide information about cell movement, interactions between cells, the activity of proteins or signalling pathways, and blood and lymphatic flow.
-
Very few cells within primary tumours are motile, and these move rapidly with an amoeboid morphology.
-
Metastatic cells are better at entering the blood vessels and withstanding shear stress than their non-metastatic counterparts.
-
Cells lodge in capillaries by various mechanisms, including physical constraint, aggregation with platelets and active interactions with the endothelium.
-
Apoptosis of tumour cells shortly after arriving at secondary sites is a major source of inefficiency in the metastatic process.
-
Certain tumour types preferentially metastasize to particular secondary sites, and the distribution of metastases cannot solely be accounted for by the pattern of blood flow. The molecular basis of this phenomenon is not well understood.
-
Imaging can be used in the preclinical evaluation of drugs that target steps in the metastatic process.
Abstract
Until recently most studies of metastasis only measured the end point of the process — macroscopic metastases. Although these studies have provided much useful information, the details of the metastatic process remain somewhat mysterious owing to difficulties in studying cell behaviour with high spatial and temporal resolution in vivo. The use of luminescent and fluorescent proteins and developments in optical imaging technology have enabled the direct observation of cancer cells spreading from their site of origin and arriving at secondary sites. This Review will describe recent advances in our understanding of the different steps of metastasis gained from cellular resolution imaging, and how these techniques can be used in preclinical drug evaluation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nature Rev. Cancer 2, 563–572 (2002).
Weiss, L. Metastatic inefficiency. Adv. Cancer Res. 54, 159–211 (1990).
Wong, C. W. et al. Apoptosis: an early event in metastatic inefficiency. Cancer Res. 61, 333–338 (2001).
Wood, S. Jr. Pathogenesis of metastasis formation observed in vivo in the rabbit ear chamber. AMA Arch. Pathol. 66, 550–568 (1958). Pioneering work imaging the behaviour of metastatic cancer cells in the rabbit ear; still holds up even after almost 50 years.
Virchow, R. Uber bewegliche thierische Zellen. Arch. Path. Anat. 28, 237–240 (1863).
Contag, C. H., Jenkins, D., Contag, P. R. & Negrin, R. S. Use of reporter genes for optical measurements of neoplastic disease in vivo. Neoplasia 2, 41–52 (2000).
Hoffman, R. M. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nature Rev. Cancer 5, 796–806 (2005). Good review article of how fluorescent proteins have been exploited for the study of cancer in vivo.
Shaner, N. C., Steinbach, P. A. & Tsien, R. Y. A guide to choosing fluorescent proteins. Nature Methods 2, 905–909 (2005).
Condeelis, J. & Segall, J. E. Intravital imaging of cell movement in tumours. Nature Rev. Cancer 3, 921–930 (2003).
Friedl, P. & Wolf, K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nature Rev. Cancer 3, 362–374 (2003).
Wang, W. et al. The activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumors. J. Cell Biol. 173, 395–404 (2006).
Kurisu, S., Suetsugu, S., Yamazaki, D., Yamaguchi, H. & Takenawa, T. Rac-WAVE2 signaling is involved in the invasive and metastatic phenotypes of murine melanoma cells. Oncogene 24, 1309–1319 (2005).
Wang, W. et al. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 64, 8585–8594 (2004).
Flesken-Nikitin, A., Williams, R. M., Zipfel, W. R., Webb, W. W. & Nikitin, A. Y. Use of multiphoton imaging for studying cell migration in the mouse. Methods Mol. Biol. 294, 335–345 (2005).
Zipfel, W. R., Williams, R. M. & Webb, W. W. Nonlinear magic: multiphoton microscopy in the biosciences. Nature Biotechnol. 21, 1369–1377 (2003).
Wyckoff, J. B., Jones, J. G., Condeelis, J. S. & Segall, J. E. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 60, 2504–2511 (2000). One of the first studies to directly observe differences in motility and interactions with blood vessels between metastatic and non-metastatic cells.
Geiger, B., Bershadsky, A., Pankov, R. & Yamada, K. M. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nature Rev. Mol. Cell Biol. 2, 793–805 (2001).
Provenzano, P. P. et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4, 38 (2006).
Wyckoff, J. B., Pinner, S. E., Gschmeissner, S., Condeelis, J. S. & Sahai, E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr. Biol. 16, 1515–1523 (2006). Analysis of the effects of anti-invasion drugs on cancer cell motility and acto–myosin organization in vivo.
Itoh, K. et al. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nature Med. 5, 221–225 (1999).
Wolf, K. et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 160, 267–277 (2003). Elegant study demonstrating how the plasticity of cancer cell movement can negate the efficacy of anti-invasion drugs.
Jiang, T. et al. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl Acad. Sci. USA 101, 17867–17872 (2004).
Bremer, C., Tung, C. H. & Weissleder, R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nature Med. 7, 743–748 (2001).
Weissleder, R., Tung, C. H., Mahmood, U. & Bogdanov, A., Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nature Biotechnol. 17, 375–378 (1999). Technically impressive study describing the development and use of a fluorescent probe that is activated in response to tumour-associated protease activity.
Tung, C. H., Mahmood, U., Bredow, S. & Weissleder, R. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 60, 4953–4958 (2000).
Wood, S. Jr. Mechanisms of establishment of tumor metastases. Pathobiol. Annu. 1, 281–308 (1971).
Sahai, E. Mechanisms of cancer cell invasion. Curr. Opin. Genet. Dev. 15, 87–96 (2005).
Sahai, E. & Marshall, C. J. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nature Cell Biol. 5, 711–719 (2003).
Wang, H. R. et al. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science 302, 1775–1779 (2003).
Sahai, E., Garcia-Medina, R., Pouyssegur, J. & Vial, E. Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility. J. Cell Biol. 176, 35–42 (2007).
DiCostanzo, D., Rosen, P. P., Gareen, I., Franklin, S. & Lesser, M. Prognosis in infiltrating lobular carcinoma. An analysis of “classical” and variant tumors. Am. J. Surg. Pathol. 14, 12–23 (1990).
Yamamoto, E., Kohama, G., Sunakawa, H., Iwai, M. & Hiratsuka, H. Mode of invasion, bleomycin sensitivity, and clinical course in squamous cell carcinoma of the oral cavity. Cancer 51, 2175–2180 (1983).
Bremer, C. et al. Optical imaging of spontaneous breast tumors using protease sensing 'smart' optical probes. Invest. Radiol. 40, 321–327 (2005).
Ahmed, F. et al. GFP expression in the mammary gland for imaging of mammary tumor cells in transgenic mice. Cancer Res. 62, 7166–7169 (2002).
Vooijs, M., Jonkers, J., Lyons, S. & Berns, A. Noninvasive imaging of spontaneous retinoblastoma pathway-dependent tumors in mice. Cancer Res. 62, 1862–1867 (2002).
Sharpless, N. E. & Depinho, R. A. The mighty mouse: genetically engineered mouse models in cancer drug development. Nature Rev. Drug Discov. 5, 741–754 (2006).
Drake, J. M., Gabriel, C. L. & Henry, M. D. Assessing tumor growth and distribution in a model of prostate cancer metastasis using bioluminescence imaging. Clin. Exp. Metastasis 22, 674–684 (2005).
Tassone, P. et al. A clinically relevant SCID-hu in vivo model of human multiple myeloma. Blood 106, 713–716 (2005).
Berking, C. & Herlyn, M. Human skin reconstruct models: a new application for studies of melanocyte and melanoma biology. Histol. Histopathol. 16, 669–674 (2001).
Wyckoff, J. et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 64, 7022–7029 (2004).
Wyckoff, J. B. et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67, 2649–2656 (2007).
Goswami, S. et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 65, 5278–5283 (2005).
Lin, E. Y. et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 163, 2113–2126 (2003).
Sahai, E. et al. Simultaneous imaging of GFP, CFP and collagen in tumors in vivo using multiphoton microscopy. BMC Biotechnol. 5, 14 (2005).
Xue, C. et al. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res. 66, 192–197 (2006).
Li, C. Y. et al. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J. Natl Cancer Inst. 92, 143–147 (2000).
Chang, Y. S. et al. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc. Natl Acad. Sci. USA 97, 14608–14613 (2000). Provocative study suggesting that tumour cells can contribute directly to tumour vasculature.
Hendrix, M. J. et al. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc. Natl Acad. Sci. USA 98, 8018–8023 (2001).
Dadiani, M. et al. Real-time imaging of lymphogenic metastasis in orthotopic human breast cancer. Cancer Res. 66, 8037–8041 (2006).
Hoshida, T. et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 66, 8065–8075 (2006).
Hagendoorn, J. et al. Onset of abnormal blood and lymphatic vessel function and interstitial hypertension in early stages of carcinogenesis. Cancer Res. 66, 3360–3364 (2006).
Carr, I. Lymphatic metastasis. Cancer Metastasis Rev. 2, 307–317 (1983).
Carr, J., Carr, I., Dreher, B. & Betts, K. Lymphatic metastasis: invasion of lymphatic vessels and efflux of tumour cells in the afferent popliteal lymph as seen in the Walker rat carcinoma. J. Pathol. 132, 287–305 (1980).
Shields, J. D. et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 11, 526–538 (2007).
Isaka, N., Padera, T. P., Hagendoorn, J., Fukumura, D. & Jain, R. K. Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function. Cancer Res. 64, 4400–4404 (2004).
Alexandrakis, G. et al. Two-photon fluorescence correlation microscopy reveals the two-phase nature of transport in tumors. Nature Med. 10, 203–207 (2004).
Cameron, M. D. et al. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 60, 2541–2546 (2000).
Tarin, D. et al. Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Cancer Res. 44, 3584–3592 (1984).
Ito, S. et al. Real-time observation of micrometastasis formation in the living mouse liver using a green fluorescent protein gene-tagged rat tongue carcinoma cell line. Int. J. Cancer 93, 212–217 (2001).
Naumov, G. N. et al. Cellular expression of green fluorescent protein, coupled with high-resolution in vivo videomicroscopy, to monitor steps in tumor metastasis. J. Cell Sci. 112, 1835–1842 (1999).
Im, J. H. et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 64, 8613–8619 (2004).
Wang, H. et al. Tumor cell α3β1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J. Cell Biol. 164, 935–941 (2004).
Tsuji, K. et al. Dual-color imaging of nuclear-cytoplasmic dynamics, viability, and proliferation of cancer cells in the portal vein area. Cancer Res. 66, 303–306 (2006).
Wong, C. W. et al. Intravascular location of breast cancer cells after spontaneous metastasis to the lung. Am. J. Pathol. 161, 749–753 (2002).
Luzzi, K. J. et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 153, 865–873 (1998).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Rafii, S. & Lyden, D. S100 chemokines mediate bookmarking of premetastatic niches. Nature Cell Biol. 8, 1321–1323 (2006).
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature Cell Biol. 8, 1369–1375 (2006).
Donato, R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 33, 637–668 (2001).
Scherbarth, S. & Orr, F. W. Intravital videomicroscopic evidence for regulation of metastasis by the hepatic microvasculature: effects of interleukin-1a on metastasis and the location of B16F1 melanoma cell arrest. Cancer Res. 57, 4105–4110 (1997).
Sipkins, D. A. et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969–973 (2005). Elegant study that uses intravital confocal imaging of the skull to observe the homing of leukaemia cells to SDF1-expressing endothelium in the bone.
Alencar, H., Mahmood, U., Kawano, Y., Hirata, T. & Weissleder, R. Novel multiwavelength microscopic scanner for mouse imaging. Neoplasia 7, 977–983 (2005).
Fidler, I. J. Metastasis: guantitative analysis of distribution and fate of tumor embolilabeled with 125 I-5-iodo-2′-deoxyuridine. J. Natl Cancer Inst. 45, 773–782 (1970).
Kim, J. W. et al. Rapid apoptosis in the pulmonary vasculature distinguishes non-metastatic from metastatic melanoma cells. Cancer Lett. 213, 203–212 (2004).
Varghese, H. J. et al. Activated ras regulates the proliferation/apoptosis balance and early survival of developing micrometastases. Cancer Res. 62, 887–891 (2002).
Frisch, S. M. & Screaton, R. A. Anoikis mechanisms. Curr. Opin. Cell Biol. 13, 555–562 (2001).
Qiu, H. et al. Arrest of B16 melanoma cells in the mouse pulmonary microcirculation induces endothelial nitric oxide synthase-dependent nitric oxide release that is cytotoxic to the tumor cells. Am. J. Pathol. 162, 403–412 (2003).
Bouvet, M. et al. In vivo color-coded imaging of the interaction of colon cancer cells and splenocytes in the formation of liver metastases. Cancer Res. 66, 11293–11297 (2006).
Pardoll, D. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 21, 807–839 (2003).
Smyth, M. J., Godfrey, D. I. & Trapani, J. A. A fresh look at tumor immunosurveillance and immunotherapy. Nature Immunol. 2, 293–299 (2001).
Boissonnas, A., Fetler, L., Zeelenberg, I. S., Hugues, S. & Amigorena, S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J. Exp. Med. 204, 345–356 (2007). This study and the one below illustrate the potential of imaging interactions between tumour and immune cells.
Mrass, P. et al. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J. Exp. Med. 203, 2749–2761 (2006).
Bonnet, D. & Dick, J. E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Med. 3, 730–737 (1997).
Bjerkvig, R., Tysnes, B. B., Aboody, K. S., Najbauer, J. & Terzis, A. J. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nature Rev. Cancer 5, 899–904 (2005).
Amoh, Y. et al. Nestin-linked green fluorescent protein transgenic nude mouse for imaging human tumor angiogenesis. Cancer Res. 65, 5352–5357 (2005).
Ma, X., Robin, C., Ottersbach, K. & Dzierzak, E. The Ly-6A (Sca-1) GFP transgene is expressed in all adult mouse hematopoietic stem cells. Stem Cells 20, 514–521 (2002).
Montarras, D. et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science 309, 2064–2067 (2005).
Naumov, G. N. et al. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 62, 2162–2168 (2002).
Naumov, G. N., MacDonald, I. C., Chambers, A. F. & Groom, A. C. Solitary cancer cells as a possible source of tumour dormancy? Semin. Cancer Biol. 11, 271–276 (2001).
Heyn, C. et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn. Reson. Med. 56, 1001–1010 (2006).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1, 571–573 (1889).
Kang, Y. et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc. Natl Acad. Sci. USA 102, 13909–13914 (2005).
Sloan, E. K. & Anderson, R. L. Genes involved in breast cancer metastasis to bone. Cell Mol. Life Sci. 59, 1491–1502 (2002).
Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001).
El-Deiry, W. S., Sigman, C. C. & Kelloff, G. J. Imaging and oncologic drug development. J. Clin. Oncol. 24, 3261–3273 (2006).
Glory, E. & Murphy, R. F. Automated subcellular location determination and high-throughput microscopy. Dev. Cell 12, 7–16 (2007).
Wolff, M., Wiedenmann, J., Nienhaus, G. U., Valler, M. & Heilker, R. Novel fluorescent proteins for high-content screening. Drug Discov. Today 11, 1054–1060 (2006).
Bouvet, M. et al. Real-time optical imaging of primary tumor growth and multiple metastatic events in a pancreatic cancer orthotopic model. Cancer Res. 62, 1534–1540 (2002).
Shah, K. & Weissleder, R. Molecular optical imaging: applications leading to the development of present day therapeutics. Neuro Rx 2, 215–225 (2005).
Weissleder, R. Molecular imaging in cancer. Science 312, 1168–1171 (2006).
van der Pluijm, G. et al. Interference with the microenvironmental support impairs the de novo formation of bone metastases in vivo. Cancer Res. 65, 7682–7690 (2005).
Hoffman, R. M. Advantages of multi-color fluorescent proteins for whole-body and in vivo cellular imaging. J. Biomed. Opt. 10, 41202 (2005).
Montet, X., Ntziachristos, V., Grimm, J. & Weissleder, R. Tomographic fluorescence mapping of tumor targets. Cancer Res. 65, 6330–6336 (2005).
Dennis, M. S. et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res. 67, 254–261 (2007).
Jain, R. K., Tong, R. T. & Munn, L. L. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 67, 2729–2735 (2007).
Shah, K., Jacobs, A., Breakefield, X. O. & Weissleder, R. Molecular imaging of gene therapy for cancer. Gene Ther. 11, 1175–1187 (2004).
Uhrbom, L., Nerio, E. & Holland, E. C. Dissecting tumor maintenance requirements using bioluminescence imaging of cell proliferation in a mouse glioma model. Nature Med. 10, 1257–1260 (2004).
Wang, Y. et al. Noninvasive indirect imaging of vascular endothelial growth factor gene expression using bioluminescence imaging in living transgenic mice. Physiol. Genomics 24, 173–180 (2006).
Wang, W. & El-Deiry, W. S. Bioluminescent molecular imaging of endogenous and exogenous p53-mediated transcription in vitro and in vivo using an HCT116 human colon carcinoma xenograft model. Cancer Biol. Ther. 2, 196–202 (2003).
Messerli, S. M. et al. A novel method for imaging apoptosis using a caspase-1 near-infrared fluorescent probe. Neoplasia 6, 95–105 (2004).
Schellenberger, E. A. et al. Optical imaging of apoptosis as a biomarker of tumor response to chemotherapy. Neoplasia 5, 187–192 (2003).
Ilagan, R. et al. Imaging mitogen-activated protein kinase function in xenograft models of prostate cancer. Cancer Res. 66, 10778–10785 (2006).
Paulmurugan, R. & Gambhir, S. S. An intramolecular folding sensor for imaging estrogen receptor-ligand interactions. Proc. Natl Acad. Sci. USA 103, 15883–15888 (2006).
Harada, H., Kizaka-Kondoh, S. & Hiraoka, M. Optical imaging of tumor hypoxia and evaluation of efficacy of a hypoxia-targeting drug in living animals. Mol. Imaging 4, 182–193 (2005).
Overall, C. M. & Kleifeld, O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nature Rev. Cancer 6, 227–239 (2006).
Brown, E. et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nature Med. 9, 796–800 (2003).
Brown, E. B. et al. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nature Med. 7, 864–868 (2001). Illustrates the many and complex types of measurements that can be made using multiphoton imaging.
Tozer, G. M. et al. Intravital imaging of tumour vascular networks using multi-photon fluorescence microscopy. Adv. Drug Deliv. Rev. 57, 135–152 (2005).
Tozer, G. M. et al. Mechanisms associated with tumor vascular shut-down induced by combretastatin A-4 phosphate: intravital microscopy and measurement of vascular permeability. Cancer Res. 61, 6413–6422 (2001).
Montet, X. et al. Tomographic fluorescence imaging of tumor vascular volume in mice. Radiology 242, 751–758 (2007).
Serganova, I. et al. Molecular imaging of temporal dynamics and spatial heterogeneity of hypoxia-inducible factor-1 signal transduction activity in tumors in living mice. Cancer Res. 64, 6101–6108 (2004).
Tong, R. T. et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 64, 3731–3736 (2004).
Winkler, F. et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6, 553–563 (2004).
Doubrovin, M., Serganova, I., Mayer-Kuckuk, P., Ponomarev, V. & Blasberg, R. G. Multimodality in vivo molecular-genetic imaging. Bioconjug. Chem. 15, 1376–1388 (2004).
Weissleder, R. & Ntziachristos, V. Shedding light onto live molecular targets. Nature Med. 9, 123–128 (2003).
Hanlon, E. B. et al. Prospects for in vivo Raman spectroscopy. Phys. Med. Biol. 45, R1–R59 (2000).
Baena, J. R. & Lendl, B. Raman spectroscopy in chemical bioanalysis. Curr. Opin. Chem. Biol. 8, 534–539 (2004).
Cheng, J. X., Jia, Y. K., Zheng, G. & Xie, X. S. Laser-scanning coherent anti-Stokes Raman scattering microscopy and applications to cell biology. Biophys. J. 83, 502–509 (2002).
Shaner, N. C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nature Biotechnol. 22, 1567–1572 (2004).
Shah, K., Tang, Y., Breakefield, X. & Weissleder, R. Real-time imaging of TRAIL-induced apoptosis of glioma tumors in vivo. Oncogene 22, 6865–6872 (2003).
Zaheer, A. et al. In vivo near-infrared fluorescence imaging of osteoblastic activity. Nature Biotechnol. 19, 1148–1154 (2001).
Frangioni, J. V. Self-illuminating quantum dots light the way. Nature Biotechnol. 24, 326–328 (2006).
So, M. K., Xu, C., Loening, A. M., Gambhir, S. S. & Rao, J. Self-illuminating quantum dot conjugates for in vivo imaging. Nature Biotechnol. 24, 339–343 (2006).
Montet, X., Montet-Abou, K., Reynolds, F., Weissleder, R. & Josephson, L. Nanoparticle imaging of integrins on tumor cells. Neoplasia 8, 214–222 (2006).
Trepel, M., Arap, W. & Pasqualini, R. In vivo phage display and vascular heterogeneity: implications for targeted medicine. Curr. Opin. Chem. Biol. 6, 399–404 (2002).
Weissleder, R., Kelly, K., Sun, E. Y., Shtatland, T. & Josephson, L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nature Biotechnol. 23, 1418–1423 (2005).
Chen, X., Conti, P. S. & Moats, R. A. In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in brain tumor xenografts. Cancer Res. 64, 8009–8014 (2004).
Cabantous, S., Terwilliger, T. C. & Waldo, G. S. Protein tagging and detection with engineered self-assembling fragments of green fluorescent protein. Nature Biotechnol. 23, 102–107 (2005).
De, A. & Gambhir, S. S. Noninvasive imaging of protein-protein interactions from live cells and living subjects using bioluminescence resonance energy transfer. FASEB J. 19, 2017–2019 (2005).
Paulmurugan, R., Massoud, T. F., Huang, J. & Gambhir, S. S. Molecular imaging of drug-modulated protein-protein interactions in living subjects. Cancer Res. 64, 2113–2119 (2004).
Bird, D. K. et al. Metabolic mapping of MCF10A human breast cells via multiphoton fluorescence lifetime imaging of the coenzyme NADH. Cancer Res. 65, 8766–8773 (2005).
Ntziachristos, V. & Chance, B. Probing physiology and molecular function using optical imaging: applications to breast cancer. Breast Cancer Res. 3, 41–46 (2001).
Zhang, H. F., Maslov, K., Stoica, G. & Wang, L. V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nature Biotechnol. 24, 848–851 (2006).
Huang, D. et al. Optical coherence tomography. Science 254, 1178–1181 (1991).
Fujimoto, J. G., Pitris, C., Boppart, S. A. & Brezinski, M. E. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia 2, 9–25 (2000).
Zharov, V. P., Galanzha, E. I., Menyaev, Y. & Tuchin, V. V. In vivo high-speed imaging of individual cells in fast blood flow. J. Biomed. Opt. 11, 054034 (2006).
Zharov, V. P., Galanzha, E. I., Shashkov, E. V., Khlebtsov, N. G. & Tuchin, V. V. In vivo photoacoustic flow cytometry for monitoring of circulating single cancer cells and contrast agents. Opt. Lett. 31, 3623–3625 (2006).
Amoh, Y. et al. Visualization of nascent tumor angiogenesis in lung and liver metastasis by differential dual-color fluorescence imaging in nestin-linked-GFP mice. Clin. Exp. Metastasis 23, 315–322 (2006).
Acknowledgements
I thank laboratory members and E. Brockbank for their comments and Cancer Research UK for funding.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary information S1 (movie)
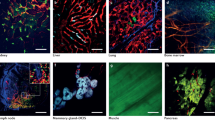
Directed movement of cancer cells towards blood vessels. The margin of a tumour is imaged to reveal intrinsic collagen, autofluorescence, reflectance signals and green fluorescent protein (GFP)-expressing melanoma cells. In merged images melanoma cells (A375) are in green, collagen in blue, autofluorescence in red (revealing some phagocytic perivascular cells), and reflective material in white (revealing blood vessels and other non-tumour cells). Movie spans 32 minutes, frame is 600microns x 600microns. (AVI 8634 kb)
Supplementary information S2 (movie)
Amoeboid cancer cell invasion in vivo. The movement of green fluorescent protein (GFP)-expressing squamous cell carcinoma cells (A431) into collagen rich matrix surrounding a tumour is shown. Carcinoma cells are in green and collagen fibres are in red. Movie spans 20 minutes, frame is 150microns x 180microns. (AVI 216 kb)
Related links
Glossary
- Intravasation
-
The entry of cells to the vasculature.
- Extravasation
-
The exit of cells from the vasculature.
- Intravital imaging
-
A generic term for imaging a living organism.
- Multiphoton microscopy
-
This type of microscopy uses multiple longer wavelength photons (usually between 700–1,000 nm) to excite a fluorophore. Has the benefit that longer wavelengths penetrate tissue better to excite fluorophores and that fluorophore excitation only occurs at the focal point, thereby reducing spurious signals from outside the focal plane. These factors combine to greatly improve the depth of tissue that can be imaged using multiphoton laser scanning microscopy.
- Confocal or laser scanning microscopy
-
Moves a focused point of laser light around the area or volume of interest and uses a 'pin-hole' to capture light specifically emitted from that point; this provides high-resolution in three dimensions.
- Amoeboid motility
-
Motility characterized by high speeds, lack of stable polarity and a relatively amorphous cell shape. Frequently exhibited by cancer cells and leukocytes in vivo.
- Acto–myosin interactions
-
Acto–myosin interactions exert force on the filamentous (F) actin cytoskeleton in cells.
- Extracellular matrix
-
(ECM). A complex structural network of proteins and carbohydrates that surrounds cells and provides support and structure to tissues.
- Second harmonic generation
-
Certain materials will emit light of exactly half the wavelength they are illuminated with. Collagen fibres are particularly suited for this phenomenon owing to large numbers of aligned α-helices.
- Matrix metalloproteinases
-
(MMPs). Proteases that cleave ECM components.
- Coherence
-
A physical technique that uses interference between a reference beam of light and light returning from the sample to determine how light is reflected by the sample.
- Xenografts
-
Tumours that result from the injection or surgical implantation of tumour cells into either a syngeneic or immunocompromised animal.
- Window chambers
-
Can be implanted in the skin of mice to enable imaging deeper inside the animal without the need for surgery at the time of imaging. They have the benefit that the same area can be repeatedly imaged over a number of days.
- Shear stress
-
The physical force exerted on cells in the blood as a result of blood flow.
- Sub-capsular region of lymph nodes
-
The area where lymph drained from the surrounding tissue enters the node.
- Pre-metastatic niche
-
An area of tissue that is particularly suited for colonization by disseminating tumour cells.
- S100 proteins
-
A family of Ca2+ EF-hand-binding proteins with a diverse range of intra- and extracellular functions. In particular, S100A8 and S100A9 have been implicated in modulation of immune cell migration.
- Anoikis
-
Apoptosis triggered by lack of attachment to a substrate.
- Vascular volume fraction
-
A measure of the volume of a tissue occupied by the vasculature.
- Multi-modal imaging
-
Multi-modal imaging agents are designed such that they can be detected by more than one imaging technique (usually optical, computed tomography, magnetic resonance imaging and/or positron emission tomography), thereby making them more versatile.
Rights and permissions
About this article
Cite this article
Sahai, E. Illuminating the metastatic process. Nat Rev Cancer 7, 737–749 (2007). https://doi.org/10.1038/nrc2229
Issue Date:
DOI: https://doi.org/10.1038/nrc2229
This article is cited by
-
Brevilin A is a potent anti-metastatic CRC agent that targets the VEGF-IL6-STAT3 axis in the HSCs-CRC interplay
Journal of Translational Medicine (2023)
-
CD271 activation prevents low to high-risk progression of cutaneous squamous cell carcinoma and improves therapy outcomes
Journal of Experimental & Clinical Cancer Research (2023)
-
Prediction of cell migration potential on human breast cancer cells treated with Albizia lebbeck ethanolic extract using extreme machine learning
Scientific Reports (2023)
-
Effect of substrate stiffness on friction in collective cell migration
Scientific Reports (2022)
-
Low lamin A levels enhance confined cell migration and metastatic capacity in breast cancer
Oncogene (2022)