Abstract
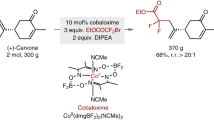
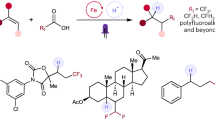
Fluorination is a reaction that is useful in improving the chemical stability and changing the binding affinity of biologically active compounds. The protocol described here can be used to replace aliphatic, C(sp3)-H hydrogen in small molecules with fluorine. Notably, isolated methylene groups and unactivated benzylic sites are accessible. The method uses readily available manganese porphyrin and manganese salen catalysts and various fluoride ion reagents, including silver fluoride (AgF), tetrabutylammonium fluoride and triethylamine trihydrofluoride (TREAT·HF), as the source of fluorine. Typically, the reactions afford 50–70% yield of mono-fluorinated products in one step. Two representative examples, the fragrance component celestolide and the nonsteroidal anti-inflammatory drug ibuprofen, are described; they produced useful isolated quantities (250–300 mg, ∼50% yield) of fluorinated material over periods of 1–8 h. The procedures are performed in a typical fume hood using ordinary laboratory glassware. No special precautions to rigorously exclude water are required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bohm, H.J. et al. Fluorine in medicinal chemistry. Chembiochem 5, 637–643 (2004).
Muller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Purser, S., Moore, P.R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008).
Ametamey, S.M., Honer, M. & Schubiger, P.A. Molecular imaging with PET. Chem. Rev. 108, 1501–1516 (2008).
Lee, E., Hooker, J.M. & Ritter, T. Nickel-mediated oxidative fluorination for PET with aqueous F-18 fluoride. J. Am. Chem. Soc. 134, 17456–17458 (2012).
Lee, E. et al. A fluoride-derived electrophilic late-stage fluorination reagent for PET imaging. Science 334, 639–642 (2011).
Schlyer, D.J. PET tracers and radiochemistry. Ann. Acad. Med. Singapore 33, 146–154 (2004).
Rozen, S. Elemental fluorine and HOF-CH3CN in service of general organic chemistry. Eur. J. Org. Chem. 2005, 2433–2447 (2005).
Ritter, T., Furuya, T. & Kamlet, A.S. Catalysis for fluorination and trifluoromethylation. Nature 473, 470–477 (2011).
Hull, K.L., Anani, W.Q. & Sanford, M.S. Palladium-catalyzed fluorination of carbon-hydrogen bonds. J. Am. Chem. Soc. 128, 7134–7135 (2006).
Bloom, S. et al. A polycomponent metal-catalyzed aliphatic, allylic, and benzylic fluorination. Angew. Chem. Int. Ed. Engl. 51, 10580–10583 (2012).
McMurtrey, K.B., Racowski, J.M. & Sanford, M.S. Pd-catalyzed C-H fluorination with nucleophilic fluoride. Org. Lett. 14, 4094–4097 (2012).
Bloom, S. et al. Iron(II)-catalyzed benzylic fluorination. Org. Lett. 15, 1722–1724 (2013).
Amaoka, Y., Nagatomo, M. & Inoue, M. Metal-free fluorination of C(sp3)-H bonds using a catalytic N-oxyl radical. Org. Lett. 15, 2160–2163 (2013).
Liu, W. et al. Oxidative aliphatic C-H fluorination with fluoride ion catalyzed by a manganese porphyrin. Science 337, 1322–1325 (2012).
Liu, W. & Groves, J.T. Manganese-catalyzed oxidative benzylic C-H fluorination by fluoride ions. Angew. Chem. Int. Ed. Engl. 52, 6024–6027 (2013).
Pangborn, A.B., Giardello, M.A., Grubbs, R.H., Rosen, R.K. & Timmers, F.J. Safe and convenient procedure for solvent purification. Organometallics 15, 1518–1520 (1996).
Acknowledgements
C-H fluorination of hydrocarbons and method development were supported by the Center for Catalytic Hydrocarbon Functionalization, an Energy Frontier Research Center, US Department of Energy, Office of Science, Basic Energy Sciences, under award no. DE SC0001298. Fluorination of biomolecules and mechanistic analyses were supported by the US National Science Foundation (CHE-1148597). Purchase of the GC-mass spectrometer was supported by the National Institutes of Health (2R37 GM036298). Partial support of this work, including the purchase of flash chromatographic equipment, was provided by Merck, Inc.
Author information
Authors and Affiliations
Contributions
W.L., X.H. and J.T.G. designed the experiments; W.L. and X.H. conducted the experiments; W.L., X.H. and J.T.G. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Liu, W., Huang, X. & Groves, J. Oxidative aliphatic C-H fluorination with manganese catalysts and fluoride ion. Nat Protoc 8, 2348–2354 (2013). https://doi.org/10.1038/nprot.2013.144
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.144
This article is cited by
-
Controlled radical fluorination of poly(meth)acrylic acids in aqueous solution
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.