Abstract
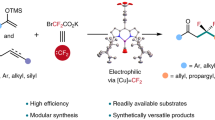
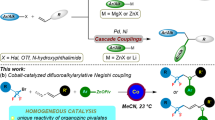
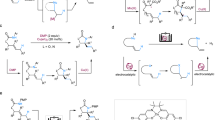
Developing practical strategies to produce high-value chemicals, such as pharmacologically active molecules, from bulk starting materials is an ongoing challenge. One potential route to this goal is the C(sp3)–H fluoroalkylation of natural products, made possible by the unique physiological properties of fluorine-containing groups. However, protocols for such fluoroalkylation remain underdeveloped. Here we report a method for the site-selective allylic fluoroalkylation of olefins through a cobalt-catalysed process comprising halogen-atom transfer and hydrogen-atom transfer. This low-cost and operationally simple protocol is readily scalable to a 2 mol scale under mild conditions. Mechanistic studies indicate that both the cobaloxime catalyst and N,N-diisopropylethylamine are essential for producing fluoroalkyl radicals. Density functional theory calculations reveal that the observed site-selectivity is controlled by steric hindrance in the cobalt-promoted hydrogen-atom transfer step.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Hong, B., Luo, T. & Lei, X. Late-stage diversification of natural products. ACS Cent. Sci. 6, 622–635 (2020).
Guillemard, L., Kaplaneris, N., Ackermann, L. & Johansson, M. J. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 5, 522–545 (2021).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83, 770–803 (2020).
Newman, D. J., Cragg, G. M. & Snader, K. M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 17, 215–234 (2000).
Jordan, M. A. & Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 4, 253–265 (2004).
Nobili, S. et al. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 59, 365–378 (2009).
Butler, M. S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 67, 2141–2153 (2004).
Clardy, J. & Walsh, C. Lessons from natural molecules. Nature 432, 829–837 (2004).
Raut, J. S. & Karuppayil, S. M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 62, 250–264 (2014).
Sharifi-Rad, J. et al. Biological activities of essential oils: from plant chemoecology to traditional healing systems. Molecules 22, 70 (2017).
Jansen, D. J. & Shenvi, R. A. Synthesis of medicinally relevant terpenes: reducing the cost and time of drug discovery. Future Med. Chem. 6, 1127–1148 (2014).
Boström, J., Brown, D. G., Young, R. J. & Keserü, G. M. Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discov. 17, 709–727 (2018).
Isanbor, C. & O’Hagan, D. Fluorine in medicinal chemistry: a review of anti-cancer agents. J. Fluorine Chem. 127, 303–319 (2006).
Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008).
Hagmann, W. K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 51, 4359–4369 (2008).
Ni, C. & Hu, J. The unique fluorine effects in organic reactions: recent facts and insights into fluoroalkylations. Chem. Soc. Rev. 45, 5441–5454 (2016).
Li, K. et al. Blue light induced difluoroalkylation of alkynes and alkenes. Org. Lett. 21, 9914–9918 (2019).
Li, K. et al. Blue light promoted difluoroalkylation of aryl ketones: synthesis of quaternary alkyl difluorides and tetrasubstituted monofluoroalkenes. Org. Lett. 22, 4261–4265 (2020).
Wang, X. et al. Copper-catalyzed C(sp3)–C(sp3) bond formation using a hypervalent iodine reagent: an efficient allylic trifluoromethylation. J. Am. Chem. Soc. 133, 16410–16413 (2011).
Parsons, A. T. & Buchwald, S. L. Copper-catalyzed trifluoromethylation of unactivated olefins. Angew. Chem. Int. Ed. 50, 9120–9123 (2011).
Xu, J. et al. Copper-catalyzed trifluoromethylation of terminal alkenes through allylic C–H bond activation. J. Am. Chem. Soc. 133, 15300–15303 (2011).
Chu, L. & Qing, F.-L. Copper-catalyzed oxidative trifluoromethylation of terminal alkenes using nucleophilic CF3SiMe3: efficient C(sp3)–CF3 bond formation. Org. Lett. 14, 2106–2109 (2012).
Barthelemy, A.-L., Bourdreux, F., Dagousset, G. & Magnier, E. Photoredox-catalyzed selective synthesis of allylic perfluoroalkanes from alkenes. Chem. Eur. J. 26, 10213–10216 (2020).
Kräutler, B. in Water Soluble Vitamins: Clinical Research and Future Application (ed. Stanger, O.) 323–346 (Springer, 2012).
Schrauzer, G. N. & Deutsch, E. Reactions of cobalt(I) supernucleophiles. The alkylation of vitamin B12s, cobaloximes(I), and related compounds. J. Am. Chem. Soc. 91, 3341–3350 (1969).
Endicott, J. F. & Netzel, T. L. Early events and transient chemistry in the photohomolysis of alkylcobalamins. J. Am. Chem. Soc. 101, 4000–4002 (1979).
Fleming, P. E., Daikh, B. E. & Finke, R. G. The coenzyme B12 derivative N1-methyl-5′-deoxyadenosylcobalamin: synthesis, characterization, stability towards Dimroth rearrangement, and Co–C thermolysis product and kinetic studies. J. Inorg. Biochem. 69, 45–57 (1998).
Li, G., Han, A., Pulling, M. E., Estes, D. P. & Norton, J. R. Evidence for formation of a Co–H bond from (H2O)2Co(dmgBF2)2 under H2: application to radical cyclizations. J. Am. Chem. Soc. 134, 14662–14665 (2012).
Weiss, M. E., Kreis, L. M., Lauber, A. & Carreira, E. M. Cobalt-catalyzed coupling of alkyl iodides with alkenes: deprotonation of hydridocobalt enables turnover. Angew. Chem. Int. Ed. 50, 11125–11128 (2011).
Branchaud, B. P., Meier, M. S. & Choi, Y. Alkyl–alkenyl cross coupling via alkyl cobaloxime radical chemistry. An alkyl equivalent to the Heck reaction compatible with common organic functional groups. Tetrahedron Lett. 29, 167–170 (1988).
Crossley, S. W. M., Barabé, F. & Shenvi, R. A. Simple, chemoselective, catalytic olefin isomerization. J. Am. Chem. Soc. 136, 16788–16791 (2014).
Wang, S. et al. Site-selective amination towards tertiary aliphatic allylamines. Nat. Catal. 5, 642–651 (2022).
Kildahl, N. K. & Viriyanon, P. Lewis acidity of cobalt(I). Inorg. Chem. 26, 4188–4194 (1987).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Yu, H. S., He, X., Li, S. L. & Truhlar, D. G. MN15: a Kohn–Sham global-hybrid exchange–correlation density functional with broad accuracy for multi-reference and single-reference systems and noncovalent interactions. Chem. Sci. 7, 5032–5051 (2016).
Shao, H., Pandharkar, R. & Cramer, C. J. Factors affecting the mechanism of 1,3-butadiene polymerization at open metal sites in Co-MFU-4l. Organometallics 41, 169–177 (2022).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Thomas, A. A. et al. Mechanistically guided design of ligands that significantly improve the efficiency of CuH-catalyzed hydroamination reactions. J. Am. Chem. Soc. 140, 13976–13984 (2018).
Constantin, T. et al. Aminoalkyl radicals as halogen-atom transfer agents for activation of alkyl and aryl halides. Science 367, 1021–1026 (2020).
Acknowledgements
This work was supported by the National Key R&D Program of China (grant number 2021YFA1500104, A.L.), the National Natural Science Foundation of China (grant number 22031008, A.L.), the Science Foundation of Wuhan (grant number 2020010601012192, A.L.), and the Postdoctoral Foundation of Hubei Province (grant number 211000025, S.W.). We thank M. Guo (WHU) and Q. Lu (WHU) for their suggestions, Shanghai Synchrotron Radiation Facility (SSRF) for XAFS testing, H. Yang (THU) and Y. Fan (Chinainstru & Quantumtech) for EPR testing with X-band EPR100, and Zhejiang Jiuzhou Pharmaceutical for 2 mol scale experiments. X.Q. acknowledges the supercomputing system in the Supercomputing Center of Wuhan University.
Author information
Authors and Affiliations
Contributions
A.L. and S.W. conceived the work. S.W. and D.R. designed the experiments and analysed the data. D.R. and S.W. performed the synthetic experiments. S.W., D.Y. and Y.G. contributed to the XAFS data. S.W. and P.W. contributed to the EPR data. Z.L. and X.Q. contributed to the DFT calculation. S.W. wrote the original manuscript which all authors revised.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Baomin Fan, Yu-hong Lam, Emmanuel Magnier and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Discussion, Tables 1–6 and Figs. 1–10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Ren, D., Liu, Z. et al. Cobalt-catalysed allylic fluoroalkylation of terpenes. Nat. Synth 2, 1202–1210 (2023). https://doi.org/10.1038/s44160-023-00365-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00365-9