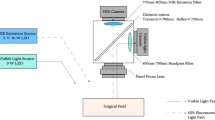
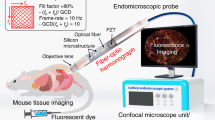
Abstract
Intravital fluorescence microscopy has emerged as a powerful technique to visualize cellular processes in vivo. However, owing to their size, the objective lenses required have limited physical accessibility to various tissue sites in the internal organs of small animals. The use of small-diameter probes using graded-index (GRIN) lenses expands the capabilities of conventional intravital microscopes to minimally invasive imaging of internal organs. In this protocol, we describe the detailed steps for the fabrication of front- and side-view GRIN probes and the integration and operation of the probes in a confocal microscope to enable visualization of fluorescent cells and microvasculature in various mouse organs. Some experience in building an optical setup is required to complete the protocol. We also present longitudinal imaging of immune cells in renal allografts and tumor development in the colon. Fabrication and integration can be completed in 5–7 h, and a typical in vivo imaging session takes 1–2 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Flusberg, B.A. et al. Fiber-optic fluorescence imaging. Nat. Meth. 2, 941–950 (2005).
Fan, Z. et al. In vivo tracking of 'color-coded' effector, natural and induced regulatory T cells in the allograft response. Nat. Med. 16, 718–722 (2010).
Kim, P. et al. In vivo wide-area cellular imaging by side-view endomicroscopy. Nat. Meth. 7, 303–305 (2010).
Kiesslich, R., Goetz, M., Vieth, M., Galle, P.R. & Neurath, M.F. Technology insight: confocal laser endoscopy for in vivo diagnosis of colorectal cancer. Nat. Clin. Pract. Oncol. 4, 480–490 (2007).
Becker, C., Fantini, M.C. & Neurath, M.F. High resolution colonoscopy in live mice. Nat. Protoc. 1, 2900–2904 (2006).
Hsiung, P.L. et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat. Med. 14, 454–458 (2008).
Dela Cruz, J.M., McMullen, J.D., Williams, R.M. & Zipfel, W.R. Feasibility of using multiphoton excited tissue autofluorescence for in vivo human histopathology. Biomed. Opt. Express 1, 1320–1330 (2010).
Waldner, M.J., Wirtz, S., Neufert, C., Becker, C. & Neurath, M.F. Confocal laser endomicroscopy and narrow-band imaging-aided endoscopy for in vivo imaging of colitis and colon cancer in mice. Nat. Protoc. 6, 1471–1481 (2011).
Barretto, R.P. et al. Time-lapse imaging of disease progression in deep brain areas using fluorescence microendoscopy. Nat. Med. 17, 223–228 (2011).
Jung, J.C. & Schnitzer, M.J. Multiphoton endoscopy. Opt. Lett. 28, 902–904 (2003).
Kim, P., Puoris'haag, M., Cote, D., Lin, C.P. & Yun, S.H. In vivo confocal and multiphoton microendoscopy. J. Biomed. Optics 13, 010501 (2008).
Miyajima, M. et al. Early acceptance of renal allografts in mice is dependent on Foxp3(+) cells. Am. J. Pathol. 178, 1635–1645 (2011).
Cole, R.W., Jinadasa, T. & Brown, C.M. Measuring and interpreting point spread functions to determine confocal microscope resolution and ensure quality control. Nat. Protoc. 6, 1929–1941 (2011).
Acknowledgements
We thank R. Colvin and C. Chase for providing the mice with renal transplantation; R. Kucherlapati and K. Hung for the mouse model of spontaneous colorectal tumor; H. Ploegh for the MHCII-GFP mice; C. Lin, D. Cote, L. Kaplan, M. Ferrari and G.Y. Koh for discussions; and D. Stevenson for proofreading of the manuscript. This work was supported by grants from the US National Institutes of Health (R21AI081010, RC1DK086242, RC2DK088661, U54CA143837, R01AI081734, P41EB015903), the US Department of Defense (FA9550-10-1-0537) and the National Research Foundation of Korea (WCU R31-2008-000-10071-0, 2009-352-C00042, 2011-0009503, WCI 2011-001).
Author information
Authors and Affiliations
Contributions
J.K.K., W.M.L., P.K., M.C. and S.H.Y. designed and wrote the protocol. K.J. and S.K. contributed figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
GRIN lens polishing device. A photograph (a) and schematic (b) of the jig used for the polishing of GRIN lenses. This device helps position the GRIN lens perpendicular to the surface of the polishing film. Different ferrule diameters can be chosen according to the diameter of the GRIN lens. The lens is loaded into the ferrule and a pushpin is loaded on top of the lens. The user can press the pushpin by hand, applying the necessary downward pressure on the lens during grinding and smoothing. The pushpin has an elastic rubber tip so that damage on the top lens surface is avoided. During polishing, the weight of the pushpin aids in sustaining a constant pressure on the lens, ensuring a regular polishing rate. As the length of the GRIN lens gets close towards the desired length, the grade of lapping polishing film should be changed from 5 µm to 1 µm to reduce the roughness of the lens surface. (PDF 1647 kb)
Supplementary Fig. 2
Technical drawing of the front-view probe holder. The front-view probe holder is used to hold the probe using a setscrew. A small blob of UV epoxy is applied to the end of the setscrew and cured, ensuring a firm grab of the optical probe without exerting excessive stress. (PDF 113 kb)
Supplementary Fig. 3
Technical drawing of the side-view probe holder. The side-view probe holder consists of four parts: custom-made stainless bearing collar, ball bearing, custom-made stainless holder and rotation pulley. (PDF 186 kb)
Supplementary Fig. 4
Drawing for assembling the side-view probe mount. The side-view probe holder is connected to a second rotation pulley via a timing belt. A rotation shaft is held in position using a set-screw in the rotation pulley. This second pulley is attached onto a cage plate with ball bearings using the collar shaft. An additional cage with ball bearing and shaft collar is used to stabilize the rotation shaft during rotation control. The viewing angle or the orientation of the prism surface is controlled by rotating the rotation shaft. (PDF 168 kb)
Supplementary Fig. 5
Cell population and motility analysis. Images of a kidney graft transplanted from a wild-type donor mouse to a FoxP3-GFP recipient mouse. Green, FoxP3+ GFP+ cells (green); red, autofluorescence from kidney capsules. Time-lapse sequence was taken every 20 seconds for 6 minutes. Track of individual cells during 6 min is overlaid onto the image taken at the end of time-lapse acquisition (Trace). The lower panel shows images zoomed at the area marked by the square. A cell population is analyzed by counting the number of cells in each FOV, and cell motility can be traced by taking time-lapse imaging. These measurements allow various dynamic processes, such as infiltration, migration, and cell-cell interaction, to be analyzed quantitatively. Scale bars, 50 µm in top panels and 20 µm in lower panels. (PDF 3076 kb)
Supplementary Video 1
Mounting the GRIN probe into a microscope. This video shows the process of mounting a side-view probe into a microscope. (MOV 877 kb)
Supplementary Video 2
Visualizing fluorescent microspheres with a GRIN probe. This video shows the process of imaging fluorescent beads with a side-view probe. (MOV 910 kb)
Supplementary Video 3
Demonstration of in vivo vasculature imaging in the murine colon with a side-view GRIN probe. This video shows the process of imaging blood vessels in the descending colon. (MOV 3330 kb)
Rights and permissions
About this article
Cite this article
Kim, J., Lee, W., Kim, P. et al. Fabrication and operation of GRIN probes for in vivo fluorescence cellular imaging of internal organs in small animals. Nat Protoc 7, 1456–1469 (2012). https://doi.org/10.1038/nprot.2012.078
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.078
This article is cited by
-
Enhanced light focusing inside scattering media with shaped ultrasound
Scientific Reports (2023)
-
Learned end-to-end high-resolution lensless fiber imaging towards real-time cancer diagnosis
Scientific Reports (2022)
-
Endoscopic vitreoretinal surgery: principles, applications and new directions
International Journal of Retina and Vitreous (2019)
-
Complex vectorial optics through gradient index lens cascades
Nature Communications (2019)
-
Label-free nanoscale optical metrology on myelinated axons in vivo
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.