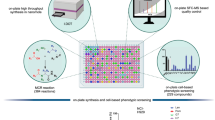
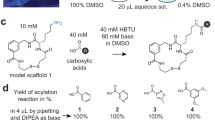
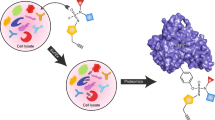
Abstract
The described oxime-based library protocol provides detailed procedures for the linkage of aminooxy functionality with aldehyde building blocks that result in the generation of libraries of multidentate inhibitors. Synthesis of inhibitors for protein tyrosine phosphatases (PTPs) and antagonists directed against the human tumor susceptibility gene 101 (TSG101) are shown as examples. Three steps are involved: (i) the design and synthesis of aminooxy platforms; (ii) tethering with aldehydes to form oxime-based linkages with sufficient purity; and (iii) direct in vitro biological evaluation of oxime products without purification. Each coupling reaction is (i) performed in capped microtubes at room temperature (20–23 °C); (ii) diluted for inhibitory evaluation; and (iii) screened with targets in microplates to provide IC50 or Kd values. The synthesis of the aminooxy platforms takes 3–5 d; tethering with the aldehydes takes 24 h; and inhibition assay of enzymes and protein-protein interactions takes 30 min and 2 h, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Zhang, Z.Y. et al. Substrate specificity of the protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 90, 4446–4450 (1993).
Zhang, Z.Y., Maclean, D., McNamara, D.J., Sawyer, T.K. & Dixon, J.E. Protein tyrosine phosphatase substrate specificity: size and phosphotyrosine positioning requirements in peptide substrates. Biochemistry 33, 2285–2290 (1994).
Jia, Z., Barford, D., Flint, A.J. & Tonks, N.K. Structural basis for phosphotyrosine peptide recognition by protein tyrosine phosphatase 1B. Science 268, 1754–1758 (1995).
Wu, L., Buist, A., den Hertog, J. & Zhang, Z.Y. Comparative kinetic analysis and substrate specificity of the tandem catalytic domains of the receptor-like protein-tyrosine phosphatase alpha. J. Biol. Chem. 272, 6994–7002 (1997).
Sarmiento, M., Zhao, Y., Gordon, S.J. & Zhang, Z.Y. Molecular basis for substrate specificity of protein-tyrosine phosphatase 1B. J. Biol. Chem. 273, 26368–26374 (1998).
Sarmiento, M. et al. Structural basis of plasticity in protein tyrosine phosphatase 1B substrate recognition. Biochemistry 39, 8171–8179 (2000).
Atwell, S., Ultsch, M., De Vos, A.M. & Wells, J.A. Structural plasticity in a remodeled protein-protein interface. Science 278, 1125–1128 (1997).
Arkin, M.R. et al. Binding of small molecules to an adaptive protein-protein interface. Proc. Natl. Acad. Sci. USA 100, 1603–1608 (2003).
Meireles, L.M. & Mustata, G. Discovery of modulators of protein-protein interactions: current approaches and limitations. Curr. Top. Med. Chem. 11, 248–257 (2011).
Tron, G.C. et al. Click chemistry reactions in medicinal chemistry: applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 28, 278–308 (2008).
Kolb, H.C., Finn, M.G. & Sharpless, K.B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. Engl. 40, 2004–2021 (2001).
Huisgen, R., Grashey, R. & Sauer, J. Chemistry of Alkenes 806–877 (Interscience, 1964).
Fazio, F., Bryan, M.C., Blixt, O., Paulson, J.C. & Wong, C.H. Synthesis of sugar arrays in microtiter plate. J. Am. Chem. Soc. 124, 14397–14402 (2002).
Srinivasan, R., Uttamchandani, M. & Yao, S.Q. Rapid assembly and in situ screening of bidentate inhibitors of protein tyrosine phosphatases. Org. Lett. 8, 713–716 (2006).
Wang, J., Uttamchandani, M., Li, J., Hu, M. & Yao, S.Q. Rapid assembly of matrix metalloprotease inhibitors using click chemistry. Org. Lett. 8, 3821–3824 (2006).
Srinivasan, R., Li, J., Ng, S.L., Kalesh, K.A. & Yao, S.Q. Methods of using click chemistry in the discovery of enzyme inhibitors. Nat. Protoc. 2, 2655–2664 (2007).
Huang, Z. et al. Derivatives of salicylic acid as inhibitors of YopH in Yersinia pestis. Chem. Biol. Drug. Des. 76, 85–99 (2010).
Zhang, X. et al. Salicylic acid based small molecule inhibitor for the oncogenic Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2). J. Med. Chem. 53, 2482–2493 (2010).
Zhou, B. et al. Targeting Mycobacterium protein tyrosine phosphatase B for antituberculosis agents. Proc. Natl. Acad. Sci. USA 107, 4573–4578 (2010).
Lee, L.V. et al. A potent and highly selective inhibitor of human α-1,3-fucosyltransferase via click chemistry. J. Am. Chem. Soc. 125, 9588–9589 (2003).
Manetsch, R. et al. In situ click chemistry: enzyme inhibitors made to their own specifications. J. Am. Chem. Soc. 126, 12809–12818 (2004).
Pagliai, F. et al. Rapid synthesis of triazole-modified resveratrol analogues via click chemistry. J. Med. Chem. 49, 467–470 (2006).
Whiting, M. et al. Inhibitors of HIV-1 protease by using in situ click chemistry. Angew. Chem. Int. Ed. Engl. 45, 1435–1439 (2006).
He, R. et al. Double click reaction for the acquisition of a highly potent and selective mPTPB inhibitor. ChemMedChem 5, 2051–2056 (2010).
Tornoe, C.W., Christensen, C. & Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 67, 3057–3064 (2002).
Wu, P. et al. Efficiency and fidelity in a click-chemistry route to triazole dendrimers by the copper(I)-catalyzed ligation of azides and alkynes. Angew. Chem. Int. Ed. Engl. 43, 3928–3932 (2004).
Malkoch, M. et al. Orthogonal approaches to the simultaneous and cascade functionalization of macromolecules using click chemistry. J. Am. Chem. Soc. 127, 14942–14949 (2005).
Aucagne, V. & Leigh, D.A. Chemoselective formation of successive triazole linkages in one pot: 'click-click' chemistry. Org. Lett. 8, 4505–4507 (2006).
Rostovtsev, V.V., Green, L.G., Fokin, V.V. & Sharpless, K.B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective 'ligation' of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 41, 2596–2599 (2002).
Dorner, S. & Westermann, B. A short route for the synthesis of 'sweet' macrocycles via a click-dimerization-ring-closing metathesis approach. Chem. Commun. (Camb) 2852–2854 (2005).
Kalia, J. & Raines, R.T. Hydrolytic stability of hydrazones and oximes. Angew. Chem. Int. Ed. Engl. 47, 7523–7526 (2008).
Liu, F. et al. A rapid oxime linker-based library approach to identification of bivalent inhibitors of the Yersinia pestis protein-tyrosine phosphatase, YopH. Bioorg. Med. Chem. Lett. 20, 2813–2816 (2010).
Bahta, M. & Burke, T.R. Jr. Oxime-based click chemistry in the development of 3-isoxazolecarboxylic acid containing inhibitors of Yersinia pestis protein tyrosine phosphatase, YopH. ChemMedChem 6, 1363–1370 (2011).
Bahta, M. et al. Utilization of nitrophenylphosphates and oxime-based ligation for the development of nanomolar affinity inhibitors of the Yersinia pestis outer protein H (YopH) phosphatase (double dagger). J. Med. Chem. 54, 2933–2943 (2011).
Liu, F., Stephen, A.G., Fisher, R.J. & Burke, T.R. Jr. Protected aminooxyprolines for expedited library synthesis: application to Tsg101-directed proline-oxime containing peptides. Bioorg. Med. Chem. Lett. 18, 1096–1101 (2008).
Liu, F. et al. SAR by oxime-containing peptide libraries: application to Tsg101 ligand optimization. Chembiochem 9, 2000–2004 (2008).
Kim, S.E. et al. Elucidation of new binding interactions with the human Tsg101 protein using modified HIV-1 Gag-p6 derived peptide ligands. ACS Med. Chem. Lett. 2, 337–341 (2011).
Tonks, N.K. Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 7, 833–846 (2006).
Blaskovich, M.A. Drug discovery and protein tyrosine phosphatases. Curr. Med. Chem. 16, 2095–2176 (2009).
Soulsby, M. & Bennett, A.M. Physiological signaling specificity by protein tyrosine phosphatases. Physiology (Bethesda) 24, 281–289 (2009).
Zhang, Z.Y. et al. The Cys(X)5Arg catalytic motif in phosphoester hydrolysis. Biochemistry 33, 15266–15270 (1994).
Zhang, Z.Y., Wang, Y. & Dixon, J.E. Dissecting the catalytic mechanism of protein-tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 91, 1624–1627 (1994).
Garrus, J.E. et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107, 55–65 (2001).
Bieniasz, P.D. Late budding domains and host proteins in enveloped virus release. Virology 344, 55–63 (2006).
Demirov, D.G., Ono, A., Orenstein, J.M. & Freed, E.O. Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc. Natl. Acad. Sci. USA 99, 955–960 (2002).
Pornillos, O. et al. Structure and functional interactions of the Tsg101 UEV domain. EMBO J. 21, 2397–2406 (2002).
Freed, E.O. The HIV-TSG101 interface: recent advances in a budding field. Trends Microbiol. 11, 56–59 (2003).
Chen, H., Liu, X., Li, Z., Zhan, P. & De Clercq, E. TSG101: a novel anti-HIV-1 drug target. Curr. Med. Chem. 17, 750–758 (2010).
Pornillos, O., Alam, S.L., Davis, D.R. & Sundquist, W.I. Structure of the Tsg101 UEV domain in complex with the PTAP motif of the HIV-1 p6 protein. Nat. Struct. Biol. 9, 812–817 (2002).
Merrifield, R.B. Solid-phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 85, 2149–2154 (1963).
Albericio, F. Solid-Phase Synthesis: A Practical Guide 1st ed. (CRC Press, 2000).
Phan, J. et al. High-resolution structure of the Yersinia pestis protein tyrosine phosphatase YopH in complex with a phosphotyrosyl mimetic-containing hexapeptide. Biochemistry 42, 13113–13121 (2003).
Liu, F. et al. Hydrazone- and hydrazide-containing N-substituted glycines as peptoid surrogates for expedited library synthesis: application to the preparation of Tsg101-directed HIV-1 budding antagonists. Org. Lett. 8, 5165–5168 (2006).
Acknowledgements
This work was supported in part by the Intramural Research Program and under the Contract No. HHSN261200800001E of the US National Institutes of Health, Center for Cancer Research, National Cancer Institute–Frederick and the National Cancer Institute, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
Author information
Authors and Affiliations
Contributions
M.B., F.L., S.-E.K. and A.G.S. carried out the experimental procedures; M.B., F.L., S.-E.K., A.G.S., R.J.F. and T.R.B. planned the work, interpreted the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bahta, M., Liu, F., Kim, SE. et al. Oxime-based linker libraries as a general approach for the rapid generation and screening of multidentate inhibitors. Nat Protoc 7, 686–702 (2012). https://doi.org/10.1038/nprot.2012.007
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.007
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.