Abstract
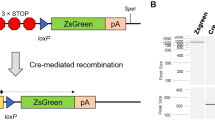
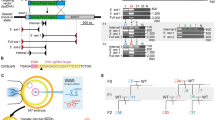
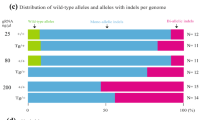
We describe here a detailed protocol for generating gene knockout rats by homologous recombination in embryonic stem (ES) cells. This protocol comprises the following procedures: derivation and expansion of rat ES cells, construction of gene-targeting vectors, generation of gene-targeted rat ES cells and, finally, production of gene-targeted rats. The major differences between this protocol and the classical mouse gene-targeting protocol include ES cell culture methods, drug selection scheme, colony picking and screening strategies. This ES cell–based gene-targeting technique allows sophisticated genetic modifications to be performed in the rat, as many laboratories have been doing in the mouse for the past two decades. Recently we used this protocol to generate Tp53 (also known as p53) gene knockout rats. The entire process requires ∼1 year to complete, from derivation of ES cells to generation of knockout rats.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Capecchi, M.R. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat. Rev. Genet. 6, 507–512 (2005).
Ying, Q.L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008).
Buehr, M. et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell 135, 1287–1298 (2008).
Li, P. et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell 135, 1299–1310 (2008).
Kawamata, M. & Ochiya, T. Generation of genetically modified rats from embryonic stem cells. Proc. Natl. Acad. Sci. USA 107, 14223–14228 (2010).
Zhao, X. et al. Derivation of embryonic stem cells from Brown Norway rats blastocysts. J. Genet. Genomics 37, 467–473 (2010).
Kobayashi, T. et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 142, 787–799 (2010).
Hirabayashi, M. et al. Establishment of rat embryonic stem cell lines that can participate in germline chimerae at high efficiency. Mol. Reprod. Dev. 77, 94 (2010).
Hirabayashi, M. et al. Rat transgenesis via embryonic stem cells electroporated with the Kusabira-orange gene. Mol. Reprod. Dev. 77, 474 (2010).
Tong, C., Li, P., Wu, N.L., Yan, Y. & Ying, Q.L. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature 467, 211–213 (2010).
Meek, S. et al. Efficient gene targeting by homologous recombination in rat embryonic stem cells. PLoS One 5, e14225 (2010).
Nguyen, H.P. et al. Behavioral abnormalities precede neuropathological markers in rats transgenic for Huntington's disease. Hum. Mol. Genet. 15, 3177–3194 (2006).
Wharram, B.L. et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol. 16, 2941–2952 (2005).
Smits, B.M., Cotroneo, M.S., Haag, J.D. & Gould, M.N. Genetically engineered rat models for breast cancer. Breast Dis. 28, 53–61 (2007).
Holmdahl, R. et al. Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol. Rev. 184, 184–202 (2001).
Cozzi, J. et al. Pronuclear DNA injection for the production of transgenic rats. In Methods in Molecular Biology Vol 561 73–88 (Humana Press, 2009).
Dann, C.T., Alvarado, A.L., Hammer, R.E. & Garbers, D.L. Heritable and stable gene knockdown in rats. Proc. Natl Acad. Sci. USA 103, 11246–11251 (2006).
van Boxtel, R., Gould, M.N., Cuppen, E. & Smits, B.M. ENU mutagenesis to generate genetically modified rat models. In Methods in Molecular Biology Vol 597 151–167 (Humana, 2010).
Zan, Y. et al. Production of knockout rats using ENU mutagenesis and a yeast-based screening assay. Nat. Biotechnol. 21, 645–651 (2003).
Izsvak, Z. et al. Generating knockout rats by transposon mutagenesis in spermatogonial stem cells. Nat. Methods 7, 443–445 (2010).
Kitada, K. et al. Transposon-tagged mutagenesis in the rat. Nat. Methods 4, 131–133 (2007).
Kitada, K., Keng, V.W., Takeda, J. & Horie, K. Generating mutant rats using the Sleeping Beauty transposon system. Methods 49, 236–242 (2009).
Mashimo, T. et al. Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases. PLoS One 5, e8870 (2010).
Menoret, S. et al. Characterization of immunoglobulin heavy chain knockout rats. Eur. J. Immunol. 40, 2932–2941 (2010).
Geurts, A.M. et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325, 433 (2009).
Cui, X. et al. Targeted integration in rat and mouse embryos with zinc-finger nucleases. Nat. Biotechnol. 29, 64–67 (2011).
Meyer, M., de Angelis, M.H., Wurst, W. & Kuhn, R. Gene targeting by homologous recombination in mouse zygotes mediated by zinc-finger nucleases. Proc. Natl. Acad. Sci. USA 107, 15022–15026 (2010).
Nagy, A., Gertsenstein, M., Vintersten, K. & Behringer, R. In Manipulating the Mouse Embryo, A Laboratory Manual 3rd edn (Cold Spring Harbor Laboratory Press, 2003).
Wu, S., Ying, G., Wu, Q. & Capecchi, M.R. A protocol for constructing gene targeting vectors: generating knockout mice for the cadherin family and beyond. Nat. Protoc. 3, 1056–1076 (2008).
Liu, P., Jenkins, N.A. & Copeland, N.G. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 13, 476–484 (2003).
Cotta-de-Almeida, V., Schonhoff, S., Shibata, T., Leiter, A. & Snapper, S.B. A new method for rapidly generating gene-targeting vectors by engineering BACs through homologous recombination in bacteria. Genome Res. 13, 2190–2194 (2003).
Sharan, S.K., Thomason, L.C., Kuznetsov, S.G. & Court, D.L. Recombineering: a homologous recombination-based method of genetic engineering. Nat. Protoc. 4, 206–223 (2009).
Gibbs, R.A. et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 428, 493–521 (2004).
Liu, X. et al. Trisomy eight in ES cells is a common potential problem in gene targeting and interferes with germ line transmission. Dev. Dyn. 209, 85–91 (1997).
Sambrook, J. & Russell, D.W. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, 2001).
Schwartzberg, P.L., Goff, S.P. & Robertson, E.J. Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science 246, 799–803 (1989).
Auerbach, W. et al. Establishment and chimera analysis of 129/SvEv- and C57BL/6-derived mouse embryonic stem cell lines. Biotechniques 29, 1024–1028, 1030, 1032 (2000).
Lemckert, F.A., Sedgwick, J.D. & Korner, H. Gene targeting in C57BL/6 ES cells. Successful germ line transmission using recipient BALB/c blastocysts developmentally matured in vitro. Nucleic Acids Res. 25, 917–918 (1997).
Schuster-Gossler, K. et al. Use of coisogenic host blastocysts for efficient establishment of germline chimeras with C57BL/6J ES cell lines. Biotechniques 31, 1022–1024, 1026 (2001).
Seong, E., Saunders, T.L., Stewart, C.L. & Burmeister, M. To knockout in 129 or in C57BL/6: that is the question. Trends Genet. 20, 59–62 (2004).
Ledermann, B. & Burki, K. Establishment of a germ-line competent C57BL/6 embryonic stem cell line. Exp. Cell Res. 197, 254–258 (1991).
Bryja, V., Bonilla, S. & Arenas, E. Derivation of mouse embryonic stem cells. Nature Protoc. 1, 2082–2087 (2006).
Kuhn, R. & Wurst, W. Gene knockout protocols. In Methods in Molecular Biology Vol 530 (Humana Press, 2009).
Acknowledgements
We thank N. Wu and Y. Yan for blastocyst injection; G. Chester for ordering rats; R. Montano and colleagues for rat husbandry; and T. Saunders for scientific input. This work was funded by a US National Institutes of Health National Center for Research Resouces grant (R01 RR025881).
Author information
Authors and Affiliations
Contributions
C.T. and Q.-L.Y. designed the study. C.T., G.H. and P.L. conducted the experiments. G.H. and C.T. wrote a draft of the paper. C.A. and Q.-L.Y. proofread and finalized the paper.
Corresponding author
Ethics declarations
Competing interests
Q.-L.Y. is an inventor on a patent relating to this study filed by the University of Edinburgh and licensed to StemCells.
Rights and permissions
About this article
Cite this article
Tong, C., Huang, G., Ashton, C. et al. Generating gene knockout rats by homologous recombination in embryonic stem cells. Nat Protoc 6, 827–844 (2011). https://doi.org/10.1038/nprot.2011.338
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.338
This article is cited by
-
Characterization of homozygous Foxn1 mutations induced in rat embryos by different delivery forms of Cas9 nuclease
Molecular Biology Reports (2023)
-
Simplification of culture conditions and feeder-free expansion of bovine embryonic stem cells
Scientific Reports (2021)
-
Differential manifestation of ocular phenotypes in TALEN-mediated p19arf knockout FVB/N and C57BL/6J mouse lines
Genes & Genomics (2020)
-
Phenotyping analysis of p53 knockout mice produced by gene editing and comparison with conventional p53 knockout mice
Genes & Genomics (2019)
-
Species generalization and differences in Hedgehog pathway regulation of fungiform and circumvallate papilla taste function and somatosensation demonstrated with sonidegib
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.