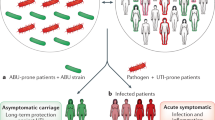
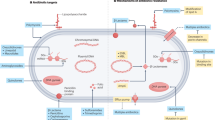
Abstract
Urinary tract infections (UTIs) inflict extreme pain and discomfort to those affected and have profound medical and socioeconomic impact. Although acute UTIs are often treatable with antibiotics, a large proportion of patients suffer from multiple recurrent infections. Here, we describe and provide a protocol for a robust murine UTI model that allows for the study of uropathogens in an ideal setting. The infections in the urinary tract can be monitored quantitatively by determining the bacterial loads at different times post-infection. In addition, the simple bladder architecture allows observation of disease progression and the uropathogenic virulence cascade using a variety of microscopic techniques. This mouse UTI model is extremely flexible, allowing the study of different bacterial strains and species of uropathogens in a broad range of mouse genetic backgrounds. We have used this protocol to identify important aspects of the host-pathogen interaction that determine the outcome of infection. The time required to complete the entire procedure will depend on the number of bacterial strains and mice included in the study. Nevertheless, one should expect 4 h of hands-on time, including inoculum preparation on the day of infection, transurethral inoculation, tissue harvest and post-harvest processing for a small group of mice (e.g., 5 mice).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Hooton, T.M. & Stamm, W.E. Diagnosis and treatment of uncomplicated urinary tract infection. Infect. Dis. Clin. North Am. 11, 551–581 (1997).
Svanborg, C. & Godaly, G. Bacterial virulence in urinary tract infection. Infect. Dis. Clin. North Am. 11, 513–529 (1997).
Wu, X.R. et al. Mammalian uroplakins. A group of highly conserved urothelial differentiation-related membrane proteins. J Biol Chem 269, 13716–13724 (1994).
Connell, H. et al. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 93, 9827–9832 (1996).
Langermann, S. et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 276, 607–611 (1997).
Mulvey, M.A. et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli . Science 282, 1494–1497 (1998).
Thankavel, K. et al. Localization of a domain in the FimH adhesin of Escherichia coli type 1 fimbriae capable of receptor recognition and use of a domain-specific antibody to confer protection against experimental urinary tract infection. J. Clin. Invest. 100, 1123–1126 (1997).
Kisielius, P.V., Schwan, W.R., Amundsen, S.K., Duncan, J.L. & Schaeffer, A.J. In vivo expression and variation of Escherichia coli type 1 and P pili in the urine of adults with acute urinary tract infections. Infect. Immun. 57, 1656–1662 (1989).
Langermann, S. et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli . J. Infect. Dis. 181, 774–778 (2000).
Nuccio, S.P. & Baumler, A.J. Evolution of the chaperone/usher assembly pathway: fimbrial classification goes Greek. Microbiol. Mol. Biol. Rev. 71, 551–575 (2007).
Thanassi, D.G. & Hultgren, S.J. Assembly of complex organelles: pilus biogenesis in gram-negative bacteria as a model system. Methods 20, 111–126 (2000).
Abraham, S.N., Sun, D., Dale, J.B. & Beachey, E.H. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature 336, 682–684 (1988).
Krogfelt, K.A., Bergmans, H. & Klemm, P. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect. Immun. 58, 1995–1998 (1990).
Martinez, J.J. & Hultgren, S.J. Requirement of Rho-family GTPases in the invasion of Type 1-piliated uropathogenic Escherichia coli . Cell Microbiol. 4, 19–28 (2002).
Martinez, J.J., Mulvey, M.A., Schilling, J.D., Pinkner, J.S. & Hultgren, S.J. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19, 2803–2812 (2000).
Zhou, G. et al. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J. Cell Sci. 114 (Pt 22): 4095–4103 (2001).
Justice, S.S. et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 101, 1333–1338 (2004).
Justice, S.S., Hunstad, D.A., Seed, P.C. & Hultgren, S.J. Filamentation by Escherichia coli subverts innate defenses during urinary tract infection. Proc. Natl. Acad. Sci. USA 103, 19884–19889 (2006).
Hopkins, W.J., Gendron-Fitzpatrick, A., Balish, E. & Uehling, D.T. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infect. Immun. 66, 2798–2802 (1998).
Schaeffer, A.J., Schwan, W.R., Hultgren, S.J. & Duncan, J.L. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect. Immun. 55, 373–380 (1987).
Bishop, B.L. et al. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat. Med. 13, 625–630 (2007).
Gunther, N.W.t., Lockatell, V., Johnson, D.E. & Mobley, H.L. In vivo dynamics of type 1 fimbria regulation in uropathogenic Escherichia coli during experimental urinary tract infection. Infect. Immun. 69, 2838–2846 (2001).
Hagberg, L. et al. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritic E. coli of human origin. Infect. Immun. 40, 273–280 (1983).
Hvidberg, H. et al. Development of a long-term ascending urinary tract infection mouse model for antibiotic treatment studies. Antimicrob. Agents Chemother. 44, 156–163 (2000).
Lane, M.C., Alteri, C.J., Smith, S.N. & Mobley, H.L. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc. Natl. Acad. Sci. USA 104, 16669–16674 (2007).
Malaviya, R., Ikeda, T., Abraham, S.N. & Malaviya, R. Contribution of mast cells to bacterial clearance and their proliferation during experimental cystitis induced by type 1 fimbriated E. coli . Immunol. Lett. 91, 103–111 (2004).
Hopkins, W.J., Hall, J.A., Conway, B.P. & Uehling, D.T. Induction of urinary tract infection by intraurethral inoculation with Escherichia coli: refining the murine model. J. Infect. Dis. 171, 462–465 (1995).
Rouschop, K.M. et al. Urothelial CD44 facilitates Escherichia coli infection of the murine urinary tract. J. Immunol. 177, 7225–7232 (2006).
Anderson, G.G. et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301, 105–107 (2003).
Mysorekar, I.U. & Hultgren, S.J. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl. Acad. Sci. USA 103, 14170–14175 (2006).
Wright, K.J., Seed, P.C. & Hultgren, S.J. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell. Microbiol. 9, 2230–2241 (2007).
Justice, S.S., Lauer, S.R., Hultgren, S.J. & Hunstad, D.A. Maturation of intracellular Escherichia coli communities requires SurA. Infect. Immun. 74, 4793–4800 (2006).
Johnson, J.R. Reflux in the mouse model of urinary tract infection. Infect. Immun. 66, 6063–6064 (1998).
Johnson, J.R. & Brown, J.J. Defining inoculation conditions for the mouse model of ascending urinary tract infection that avoid immediate vesicoureteral reflux yet produce renal and bladder infection. J. Infect. Dis. 173, 746–749 (1996).
Rosen, D.A., Hung, C.S., Kline, K.A. & Hultgren, S.J. Streptozocin-induced diabetic mouse model of urinary tract infection. Infect. Immun. 76, 4290–4298 (2008).
Garofalo, C.K. et al. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect. Immun. 75, 52–60 (2007).
Mulvey, M.A., Schilling, J.D. & Hultgren, S.J. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69, 4572–4579 (2001).
Schilling, J.D., Martin, S.M., Hung, C.S., Lorenz, R.G. & Hultgren, S.J. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli . Proc. Natl. Acad. Sci. USA 100, 4203–4208 (2003).
Rosen, D.A., Hooton, T.M., Stamm, W.E., Humphrey, P.A. & Hultgren, S.J. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4, e329 (2007).
Kau, A.L., Hunstad, D.A. & Hultgren, S.J. Interaction of uropathogenic Escherichia coli with host uroepithelium. Curr. Opin. Microbiol. 8, 54–59 (2005).
Rosen, D.A. et al. Molecular variations in Klebsiella pneumoniae and Escherichia coli FimH affect function and pathogenesis in the urinary tract. Infect. Immun. 76, 3346–3356 (2008).
Rosen, D.A. et al. Utilization of an intracellular bacterial community pathway in Klebsiella pneumoniae urinary tract infection and the effects of FimK on type 1 pilus expression. Infect. Immun. 76, 3337–3345 (2008).
Institute of Laboratory Animal Resources, Commission on Life Sciences, National research Council. Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC, USA, 1996).
Wellens, A. et al. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PLoS ONE 3, e2040 (2008).
Kulesus, R.R., Diaz-Perez, K., Slechta, E.S., Eto, D.S. & Mulvey, M.A. Impact of the RNA chaperone Hfq on the fitness and virulence potential of uropathogenic Escherichia coli . Infect. Immun. 76, 3019–3026 (2008).
Hannan, T.J. et al. LeuX tRNA-dependent and -independent mechanisms of Escherichia coli pathogenesis in acute cystitis. Mol. Microbiol. 67, 116–128 (2008).
Wright, K.J., Seed, P.C. & Hultgren, S.J. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 73, 7657–7668 (2005).
Mysorekar, I.U., Mulvey, M.A., Hultgren, S.J. & Gordon, J.I. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli . J. Biol. Chem. 277, 7412–7419 (2002).
Schilling, J.D., Lorenz, R.G. & Hultgren, S.J. Effect of trimethoprim–sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli . Infect. Immun. 70, 7042–7049 (2002).
Acknowledgements
We thank all current and former Hultgren lab members in optimizing and perfecting the murine UTI model. We thank Dr. Wandy Beatty in the Molecular Microbiology Imaging Facility at Washington University School of Medicine for her excellent technical assistance with fluorescence microscopy. C.-S.H., K.W.D. and S.J.H. are supported by National Institutes of Health Office of Research on Women's Health: Specialized Center of Research on Sex and Gender Factors Affecting Women's Health, grant R01 DK64540, National Institute of Diabetes and Digestive and Kidney Diseases, grants R01 DK051406 and National Institute of Allergy and Infectious Diseases, grants R01 AI29549, R01 AI48689 and R01 AI50011.
Author information
Authors and Affiliations
Contributions
C.-S.H., K.W.D. and S.J.H. wrote the manuscript. C.-S.H. generated the sample data presented in Anticipated Results.
Corresponding author
Rights and permissions
About this article
Cite this article
Hung, CS., Dodson, K. & Hultgren, S. A murine model of urinary tract infection. Nat Protoc 4, 1230–1243 (2009). https://doi.org/10.1038/nprot.2009.116
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.116
This article is cited by
-
Fabrication of levofloxacin-loaded porcine acellular dermal matrix hydrogel and functional assessment in urinary tract infection
Journal of Nanobiotechnology (2024)
-
Uropathogenic Escherichia coli subverts mitochondrial metabolism to enable intracellular bacterial pathogenesis in urinary tract infection
Nature Microbiology (2022)
-
17β-estradiol ameliorates delirium-like phenotypes in a murine model of urinary tract infection
Scientific Reports (2022)
-
The Effect of Air Plasma Activated Liquid on Uropathogenic Bacteria
Plasma Chemistry and Plasma Processing (2022)
-
Interleukin-6 mediates delirium-like phenotypes in a murine model of urinary tract infection
Journal of Neuroinflammation (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.