Abstract
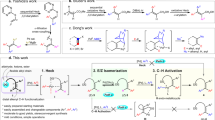
This protocol describes a diastereoselective approach for the synthesis of complex molecular architectures containing two stereogenic centers in a 1,4 relationship, one of which being an all-carbon quaternary stereogenic center. Such molecules could be intermediates in the synthesis of steroids, for example. Conceived as a single-flask synthetic sequence from ω-ene cyclopropanes, the protocol involves a concerted allylic C–H and C–C bond activation promoted by the Negishi reagent (Cp2Zr(η2-butene)). This zirconium-promenade-based procedure affords bifunctionalized products in high diastereomeric ratios after reaction of ω-ene cyclopropanes with the Negishi complex, followed by a thermal treatment and sequential addition of two different electrophiles. The method proves to be particularly efficient when carbonyl compounds are used as first electrophiles and hydrogen or elemental halides are used as second electrophiles. In addition, it offers the opportunity to create new C–C bonds via remote functionalization of a (sp3)–C–H bond, a result of a copper or copper/palladium transmetalation step that extends the scope of the process to alkyl, acyl and aromatic halide compounds as second electrophiles. The typical described protocol allows the synthesis of the highly diastereo-enriched 2-((1R*,2S*)-2-butyl-2 propylcyclopropyl)ethanol and may provide a new entry to access complex molecular segments of natural products such as steroids or C30 botryococcene. It requires a simple reaction setup and takes ∼18.5 h to run the reaction and 2 h for isolation and purification.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Labinger, J.A. & Bercaw, J.E. Understanding and exploiting C-H bond activation. Nature 417, 507–514 (2002).
Godula, K. & Sames, D. C-H bond functionalization in complex organic synthesis. Science 312, 67–72 (2006).
Shilov, A.E. & Shul'pin, G.B. Activation of C-H bonds by metal complexes. Chem. Rev. 97, 2879–2932 (1997).
Souillart, L. & Cramer, N. Catalytic C-C bond activations via oxidative addition to transition metals. Chem. Rev. 115, 9410–9464 (2015).
Chen, F., Wang, T. & Jiao, N. Recent advances in transition-metal-catalyzed functionalization of unstrained carbon-carbon bonds. Chem. Rev. 114, 8613–8661 (2014).
Rybtchinski, B. & Milstein, D. Metal insertion into C-C bonds in solution. Angew. Chem. Int. Ed. 38, 870–883 (1999).
Wencel-Delord, J. & Glorius, F. C-H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem. 5, 369–375 (2013).
Gutekunst, W.R. & Baran, P.S. C-H functionalization logic in total synthesis. Chem. Soc. Rev. 40, 1976–1991 (2011).
Giri, R., Shi, B.-F., Engle, K.M., Maugel, N. & Yu, J.-Q. Transition metal-catalyzed C-H activation reactions: diastereoselectivity and enantioselectivity. Chem. Soc. Rev. 38, 3242–3272 (2009).
Ritleng, V., Sirlin, C. & Pfeffer, M. Ru-, Rh-, and Pd-catalyzed C-C bond formation involving C-H activation and addition on unsaturated substrates: reactions and mechanistic aspects. Chem. Rev. 102, 1731–1769 (2002).
Wentzel, M.T., Reddy, V.J., Hyster, T.K. & Douglas, C.J. Chemoselectivity in catalytic C-C and C-H bond activation: controlling intermolecular carboacylation and hydroarylation of alkenes. Angew. Chem. Int. Ed. 48, 6121–6123 (2009).
Masarwa, A. & Marek, I. Selectivity in metal-catalyzed carbon-carbon bond cleavage of alkylidenecyclopropanes. Chem. Eur. J. 16, 9712–9721 (2010).
Jun, C.H. Transition metal-catalyzed carbon-carbon bond activation. Chem. Soc. Rev. 33, 610–618 (2004).
Jun, C.-H., Moon, C.W. & Lee, D.Y. Chelation-assisted carbon-hydrogen and carbon-carbon bond activation by transition metal catalysts. Chem. Eur. J. 8, 2422–2428 (2002).
Tobisu, M., Kinuta, H., Kita, Y., Re´mond, E. & Chatani, N. Rhodium(I)-catalyzed borylation of nitriles through the cleavage of carbon-cyano bonds. J. Am. Chem. Soc. 134, 115–118 (2012).
Pusterla, I. & Bode, J.W. The mechanism of the α-ketoacid-hydroxylamine amide-forming ligation. Angew. Chem. Int. Ed. 51, 513–516 (2012).
He, C., Gou, S., Huang, L. & Lei, A. Copper catalyzed arylation/C-C bond activation: an approach toward α-aryl ketones. J. Am. Chem. Soc. 132, 8273–8275 (2010).
Seiser, T. & Cramer, N. Rhodium-catalyzed C-C bond cleavage: construction of acyclic methyl substituted quaternary stereogenic centers. J. Am. Chem. Soc. 132, 5340–5341 (2010).
Winter, C. & Krause, N. Rhodium(I)-catalyzed enantioselective C-C bond activation. Angew. Chem. Int. Ed. 48, 2460–2462 (2009).
Bart, S.C. & Chirik, P.J. Selective, catalytic carbon-carbon bond activation and functionalization promoted by late transition metal catalysts. J. Am. Chem. Soc. 125, 886–887 (2003).
Marek, I., Masarwa, A., Delaye, P.-O. & Leibeling, M. Selective carbon-carbon bond cleavage for the stereoselective synthesis of acyclic systems. Angew. Chem. Int. Ed 54, 414–429 (2015).
Matsuda, T. & Yuihara, I. A rhodium(I)-catalysed formal intramolecular C-C/C-H bond metathesis. Chem. Commun. 51, 7393–7396 (2015).
Wu, J.-Q. et al. Rhodium(III)-catalyzed C-H/C-C activation sequence: vinylcyclopropanes as versatile synthons in direct C-H allylation reactions. Chem. Commun. 51, 77–80 (2015).
Masarwa, A., Weber, M. & Sarpong, R. Selective C-C and C-H bond activation/cleavage of pinene derivatives: synthesis of enantiopure cyclohexenone scaffolds and mechanistic insights. J. Am. Chem. Soc. 137, 6327–6334 (2015).
Liang, Y., Liang, Y.-F. & Jiao, N. Cu- or Fe-catalyzed C-H/C-C bond nitrogenation reactions for the direct synthesis of N-containing compounds. Org. Chem. Front. 2, 403–415 (2015).
Bhunia, S.K., Polley, A., Natarajan, R. & Jana, R. Through-space 1,4-palladium migration and 1,2-aryl shift: direct access to dibenzo[a,c]carbazoles through a triple C-H functionalization cascade. Chem. Eur. J. 21, 16786–16791 (2015).
Kou, X. et al. Copper-catalyzed aromatic C-H bond cyanation by C-CN bond cleavage of inert acetonitrile. Chem. Eur. J. 19, 16880–16886 (2013).
Sun, M., Shen, G. & Bao, W. Regioselective cleavage of unstrained C-C bond and C-H bond: palladium-copper catalyzed deacetophenonylative arylation of coumarin derivatives. Adv. Synth. Catal. 354, 3468–3474 (2012).
Matsuda, T., Suda, Y. & Takahashi, A. Double 1,4-rhodium migration cascade in rhodium-catalysed arylative ring-opening/spirocyclisation of (3-arylcyclobutylidene)acetate. Chem. Commun. 48, 2988–2990 (2012).
Zhou, W., Li, H. & Wang, L. Direct carbo-acylation reactions of 2 arylpyridines with α-diketones via Pd-catalyzed C-H activation and selective C(sp2)-C(sp2) cleavage. Org. Lett. 14, 4594–4597 (2012).
Peng, X., Zhu, Y., Ramirez, T.A., Zhao, B. & Shi, Y. New reactivity of oxaziridine: Pd(II)-catalyzed aromatic C-H ethoxycarbonylation via C-C bond cleavage. Org. Lett. 13, 5244–5247 (2011).
Wang, C., Rakshit, S. & Glorius, F. Palladium-catalyzed intermolecular decarboxylative coupling of 2-phenylbenzoic acids with alkynes via C-H and C-C bond activation. J. Am. Chem. Soc. 132, 14006–14008 (2010).
Seiser, T., Roth, O.A. & Cramer, N. Enantioselective synthesis of indanols from tert-cyclobutanols using a rhodium-catalyzed C-C/C-H activation sequence. Angew. Chem. Int. Ed. 48, 6320–6323 (2009).
Aïssa, C. & Fürstner, A. Rhodium-catalyzed C-H activation/cycloisomerization tandem. J. Am. Chem. Soc. 129, 14836–14837 (2007).
Park, Y.J., Park, J.-W. & Jun, C.-H. Metal-organic cooperative catalysis in C-H and C-C bond activation and its concurrent recovery. Acc. Chem. Res. 41, 222–234 (2008).
Chinkov, N., Majumdar, S. & Marek, I. Stereoselective preparation of dienyl zirconocene complexes via a tandem allylic C-H bond activation-elimination sequence. J. Am. Chem. Soc. 125, 13258–13264 (2003).
Chinkov, N., Levin, A. & Marek, I. Unsaturated fatty alcohol derivatives as a source of substituted allylzirconocene. Angew. Chem. Int. Ed. 45, 465–468 (2006).
Masarwa, A. et al. Merging allylic carbon-hydrogen and selective carbon-carbon bond activation. Nature 505, 199–203 (2014).
Pulis, A.P. & Aggarwal, V.K. Synthesis of enantioenriched tertiary boronic esters from secondary allylic carbamates. Application to the synthesis of C30 botryococcene. J. Am. Chem. Soc. 134, 7570–7574 (2012).
White, J.D., Reddy, G.N. & Spessard, G.O. Total synthesis of (-)-botryococcene. J. Am. Chem. Soc. 110, 1624–1626 (1988).
Deng, Z.-P., Sun, L.-R., Ji, M. & Yuan, Y.-M. Steroids from Bovistella radicata (Mont.) Pat. Biochem. Syst. Ecol. 35, 700–703 (2007).
Maestro, M.A. et al. Synthesis and biological activity of the dihydrotachysterol2 metabolite 25-hydroxydihydrotachysterol2 . J. Steroid. Biochem. Molec. Biol. 43, 359–361 (1992).
Chinkov, N., Levin, A. & Marek, I. A zirconium promenade - an efficient tool in organic synthesis. Synlett, 501–514 (2006).
Jiao, L. & Yu, Z.-X. Vinylcyclopropane derivatives in transition-metal-catalyzed cycloadditions for the synthesis of carbocyclic compounds. J. Org. Chem. 78, 6842–6848 (2013).
Vasse, J.-L. & Szymoniak, J. Access to functionalized cyclopropylcarbinyl compounds from homoallylic ethers via zirconocene intermediates. Tetrahedron Lett. 45, 6449–6451 (2004).
Goldschmidt, Z. & Crammer, B. Vinylcyclopropane rearrangements. Chem. Soc. Rev. 17, 229–267 (1988).
Harada, S., Kiyono, H., Nishio, R., Taguchi, T. & Hanzawa, Y. Complexation of vinylcyclopropanes with zirconocene-1-butene complex: application to the stereocontrolled synthesis of steroidal side chains. J. Org. Chem. 62, 3994–4001 (1997).
Dimmock, P.W. & Whitby, R.J. Zirconium-mediated ring opening of cyclopropanes. J. Chem. Soc. Chem. Commun. 2323–2324 (1994).
Luker, T. & Whitby, R.J. Organozirconium methods for the efficient construction of the bicyclo[9.3.0]tetradecane dolabellane skeleton. Tetrahedron Lett. 37, 7661–7664 (1996).
Negishi, E. & Takahashi, T. Organozirconium compounds in organic synthesis. Synthesis 1–19 (1988).
Luker, T. & Whitby, R.J. Elaboration of zirconacyclopentanes by sequential insertion of lithium chloroallylide and ketones or aldehydes. Tetrahedron Lett. 35, 785–788 (1994).
Vasseur, A., Perrin, L., Eisenstein, O. & Marek, I. Remote functionalization of hydrocarbons with reversibility enhanced stereocontrol. Chem. Sci. 6, 2770–2776 (2015).
Wipf, P. & Jahn, H. Synthetic applications of organochlorozirconocene complexes. Tetrahedron 52, 12853–12910 (1996).
Farhat, S., Zouev, I. & Marek, I. From vinyl sulfides, sulfoxides and sulfones to vinyl zirconocene derivatives. Tetrahedron 60, 1329–1337 (2004).
Chinkov, N., Majumdar, S. & Marek, I. Stereoselective synthesis and reactivity of dienyl zirconocene derivatives. Synthesis 2004, 2411–2417 (2004).
Mei, T.-S., Patel, H.H. & Sigman, M.S. Enantioselective construction of remote quaternary stereocentres. Nature 508, 340–344 (2014).
Didier, D. et al. Modulable and highly diastereoselective carbometalations of cyclopropenes. Chem. Eur. J. 20, 1038–1048 (2014).
Lebel, H., Marcoux, J.-F., Molinaro, C. & Charette, A.B. Stereoselective cyclopropanation reactions. Chem. Rev. 103, 977–1050 (2003).
Charette, A.B., Juteau, H., Lebel, H. & Molinaro, C. Enantioselective cyclopropanation of allylic alcohols with dioxaborolane ligands: scope and synthetic applications. J. Am. Chem. Soc. 120, 11943–11952 (1998).
Mandès, C., Renard, S., Rofoo, M., Roux, M.C. & Rousseau, G. Preparation of oxocanes by electrophilic cyclizations of unsaturated alcohols in the presence of bis(collidine)halonium(I) hexafluorophosphates. Eur. J. Org. Chem. 2003, 463–471 (2003).
Mascitti, V. & Corey, E.J. Enantioselective synthesis of pentacycloanammoxic acid. J. Am. Chem. Soc. 128, 3118–3119 (2006).
Kotsuki, H., Kadota, I. & Ochi, M. A novel carbon-carbon bond-forming reaction of triflates with copper(I)-catalyzed Grignard reagents. A new concise and enantiospecific synthesis of (+)-exo-brevicomin, (5R,6S)-(−)-6-acetoxy-5-hexadecanolide, and L-factor. J. Org. Chem. 55, 4417–4422 (1990).
Minko, Y., Pasco, M., Lercher, L. & Marek, I. Stereodefined trisubstituted enolates as a unique entry to all-carbon quaternary stereogenic centers in acyclic systems. Nat. Protoc. 8, 749–754 (2013).
Woodward, R.B. & Hoffman, R. The conservation of orbital symmetry. Angew. Chem. Int. Ed. Engl. 8, 781–853 (1969).
Acknowledgements
This research was supported by the European Research Council under the European Community's Seventh Framework Program (ERC grant agreement no. 338912). We thank Z. Nairoukh and R. Liu for their assistance and particularly for taking the pictures.
Author information
Authors and Affiliations
Contributions
A.V. planned, conducted and analyzed the experiments. I.M. conceived and directed the project, and wrote the manuscript, with contributions from A.V. Both authors contributed to discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Vasseur, A., Marek, I. Merging allylic C–H bond activation and C–C bond cleavage en route to the formation of a quaternary carbon stereocenter in acyclic systems. Nat Protoc 12, 74–87 (2017). https://doi.org/10.1038/nprot.2016.161
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.161
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.