Abstract
Deep brain stimulation (DBS) as a putative approach for treatment-resistant depression (TRD) has now been researched for about a decade. Several uncontrolled studies—all in relatively small patient populations and different target regions—have shown clinically relevant antidepressant effects in about half of the patients and very recently, DBS to a key structure of the reward system, the medial forebrain bundle, has yielded promising results within few days of stimulation and at much lower stimulation intensities. On the downside, DBS procedures in regions are associated with surgical risks (eg, hemorrhage) and psychiatric complications (suicidal attenuation, hypomania) as well as high costs. This overview summarizes research on the mechanisms of brain networks with respect to psychiatric diseases and—as a novelty—extrapolates to the role of the reward system in DBS for patients with treatment-resistant depression. It further evaluates relevant methodological aspects of today’s research in DBS for TRD. On the scientific side, the reward system has an important yet clearly under-recognized role in both neurobiology and treatment of depression. On the methodological side of DBS research in TRD, better animal models are clearly needed to explain clinical effects of DBS in TRD. Larger sample sizes, long-term follow-up and designs including blinded sham control are required to draw final conclusions on efficacy and side effects. Practical research issues cover study design, patient tracking, and the discussion of meaningful secondary outcome measures.
Similar content being viewed by others
INTRODUCTION
Major depression is a disorder that taxes many patients with their lives (Blair-West et al, 1999) and all with their quality of life (QoL) (Whiteford et al, 2013). The impact of this disorder on individual patients has not been lost on physicians who over history tried to develop treatments with sustained antidepressant efficacy. These efforts were not without positive outcomes—today the majority of depressed patients respond to combinations of psychotherapy and pharmacotherapy, in more resistant cases electroconvulsive therapy adds considerable benefit (Lisanby, 2007). FDA-approved neuromodulatory treatments for treatment-resistant depression (TRD) are vagus nerve stimulation, transcranial magnetic stimulation (TMS) and only very recently deep TMS (Lozano and Lipsman, 2013; Schlaepfer et al, 2010).
There is however, a sizable proportion of patients who do not benefit significantly from currently available treatments. Given the intense suffering of patients and the lack of efficacious treatments, it is understandable that many invasive and desperate treatments for psychiatric disorders were tried without scientific hypotheses and evaluation (Hariz et al, 2010). In 1937, electroconvulsive therapy was introduced by Cerletti and Bini initially as a treatment for psychotic patients (Shorter and Healy, 2007), a treatment which—by way of thorough scientific evaluation—developed into perhaps the most effective methods for TRD (Doshi, 2011).
Lesional psychosurgery for mental disorders—including anxiety disorders and depression—was promoted in the mid-1930s by Moniz with help of the neurosurgeon Lima (Moniz, 1994). Lesion surgery utilizing a stereotactic technology evolved in the mid-1940s. Scientists saw a need for more confined and less-destructive focal lesions than the ones created with the frontal lobotomy procedures that had been introduced by Moniz and were later further developed and clearly too deliberately applied by the neurologist Walter Freeman. This very need for circumscribed lesions in a first step led to the development of the stereotactic dorsomedial thalamotomy (Hariz et al, 2010; Spiegel et al, 1947) after the thalamus had been identified as a target structure with degeneration studies on postmortem brains from frontal lobotomy. At that time, Papez had just recently published his seminal paper that essentially summarized previous work and localized emotions to specific brain regions (Meyer et al, 1947; Papez, 1995). In the following decades, other stereotactic lesion surgeries were developed, which were among other disorders applied for the treatment of anxieties and depression: (1) anterior capsulotomy, (2) anterior cingulotomy (Chang et al, 2013, 3) subcaudate tractotomy, and (4) limbic leucotomy (essentially a combination of (2) and (3)). Limbic leucotomy probably had the best outcome (Coenen and Honey, 2009a). These lesion surgeries were performed until the mid of the 1990s at different centers and are still in place at certain very experienced institutions (Hurwitz et al, 2012) although most centers for good or bad reasons today have converted to the reversible, adjustable, and more benign DBS technology. Only recently the role of the reward system even in the in these historical and lesional surgical approaches were positively evaluated in a tractography study. The efficacy of all these approaches is at least in part based on their modulating action on the reward system (Schoene-Bake et al, 2010).
It was not until after 1950 that technical development made it possible to chronically stimulate human brains through implanted electrodes (Hariz et al, 2010; Miocinovic et al, 2013). Several scientists were independently exploring the potential of brain stimulation for psychiatric diseases, see Hariz et al (2010) for a detailed review of historical DBS research and Miocinovic et al (2013) for a discussion on mechanisms of DBS. Heath and colleagues described the concept of electrical self-stimulation in the human (Bishop et al, 1963; Heath, 1954). Patients and subjects stimulated at the ‘septal area’ (close to nucleus accumbens and thus in the rewards system) described this stimulation as ‘pleasant’ or ‘euphoric’ (see eg Bishop et al, 1963). This manipulation of emotions was suggested by the authors as treatment for intractable psychiatric disorders (Hariz et al, 2010). However, the lack of scientific rationale and the denial of scientific and ethical standards of their time left the Tulane University’s research later being judged as highly dubious (Baumeister, 2000).
The dream of unlimited control over brain processes using electric currents was expressed by Delgado (1971), who believed autonomic and somatic functions, behavior and emotional and mental reactions could be manipulated by electrical stimulation of specific brain areas. This enthusiasm is still shared by some of today’s researchers who believe that the possibility to manipulate human brain function ‘might well shape history as powerfully as the development of metallurgy in the Iron Age, mechanization in the Industrial Revolution or genetics in the second half of the twentieth century’ (Farah et al, 2004). This might reflect a culture in which we conceptualize our minds and bodies as machines whose dysfunctions can be fixed or substituted with technology—in most cases even without knowing about the mechanisms that are causing the symptoms. In spite of these dreams, a variety of available treatment options and novel avenues of interventions in research (Holtzheimer et al, 2012a; Schlaepfer et al, 2010), a third of patients suffering from depression can be classified as treatment-resistant (Rush et al, 2006), with very little hope of recovery, highly stigmatized and unbearably low QoL. For these patients, deep brain stimulation (DBS) is currently under investigation.
DEPRESSION NEUROBIOLOGY
Traditional treatment perspectives conceptualize depression as a general brain dysfunction by targeting hypothesized monoaminergic synaptic dysfunction (Crupi et al, 2011). More complete and appropriate treatments are thought to arise from correlating disease symptoms with dysfunctions of specific brain networks mediating mood and reward responses (Berton and Nestler, 2006; Krishnan and Nestler, 2008). This conceptualization leads to novel and testable hypotheses about targeted neuromodulatory interventions. Long-term data on DBS for depression have been recently reported on targeting the subgenual cingulate Cortex (Cg25) target (Coenen et al, 2011; Lozano et al, 2008; Mayberg et al, 2005; Puigdemont et al, 2011), the anterior limb of the internal capsule (ALIC) (Malone et al, 2009) and the nucleus accumbens septi (NAcc) (Bewernick et al, 2012; Bewernick et al, 2010). These studies, although limited in their generalizability due to small sample sizes (n<20) and missing sham control, have lead to a new enthusiasm regarding significant antidepressant results. In a merely serendipitous fashion, the use of diffusion tensor magnetic resonance imaging (DTI) tractography allowed the explanation of psychotropic side effects of DBS to the subthalamic nucleus in Parkinson’s disease (Coenen et al, 2009b). These novel studies that combined DBS with tractographic anatomy consequently led to a necessary and novel description of human reward system anatomy (Coenen et al, 2012). This whole line of research moved the reward system as a key network for stereotactic interventions into the focus of scientists’ attention (Coenen et al, 2011) (Schoene-Bake et al, 2010). Only recently studies on optogenetic neuromodulation and fast cycling voltammetry (Howe et al, 2013; Russo and Nestler, 2013) confirm this previously addressed role of the reward system and now helps to better appreciate its role in depression genesis and interventional approaches.
DBS TARGETS AND HYPOTHESES
Subgenual Cingulate White Matter (Brodman Area Cg25)
In an elegant model, the rostral cingulate cortex has been implicated to have a dominant role in regulating a corticolimbic network (Mayberg, 1997). It has been demonstrated that depression is associated with increased activity in the subgenual cingulate cortex (covering Cg25, Cg24, BA10) and remission was associated with a reduction of hypermetabolism in this region (Fily et al, 2011). Dysfunctional connections from the cingulate cortex to the dorsal (including the dorsolateral prefrontal cortex (PFC), inferior parietal cortex, and striatum) and ventral parts (hypothalamic–pituitary–adrenal axis, insula, subgenual cingulate, and brainstem) of the emotion regulation circuit in depression are involved in depression (Riva-Posse et al, 2013). It was hypothesized that DBS to the cingulate cortex would lead to antidepressant effects by modulating the depression network through a reduction of Cg25 activity (Mayberg, 1997).
Anterior Limb of The Capsula Interna
The cortico-striato-thalamo-cortical network has an important role in obsessive-compulsive disorder (OCD) (Bourne et al, 2012). Observations from lesion studies (Lippitz et al, 1999; Lipsman et al, 2007; Nuttin et al, 1999)and antidepressant effects that were seen in OCD patients who were stimulated in the ALIC/the ventral striatum (Greenberg et al, 2008), lead to a study in which this structure was stimulated in TRD (Malone et al, 2009).
Targets in the Reward System: NAcc and MFB
Predictions about anticipated future rewarding events are encoded in dopamine concentrations in the ventral striatum. We now learn that the amount of dopamine itself encodes the distance from the reward (Howe et al, 2013). The reward system itself is obviously more concerned with the reward anticipation than with the consummatory phase of reward. Recent data suggest dysfunctions of structures implicated in the human reward system in mood disorders, particularly in the ventral tegmental area (VTA), the nucleus accumbens (NAcc and the pathways associated with them (medial forebrain bundle (MFB)) (Russo and Nestler, 2013). These facts now and in retrospect render these structures as promising targets for neuromodulatory interventions with putative anti-anhedonic and motivational effects after tractographic research had earlier already anticipated these clinical effects (Coenen et al, 2011).
Nucleus Accumbens septi
NAcc is another structure that has been identified as a key center of the depression network (Berton and Nestler, 2006). Specifically, the NAcc is thought to act as the motivation gateway between systems involved in emotion and motor control (Schlaepfer et al, 2008). Anhedonia, which has been correlated with NAcc dysfunction (Pizzagalli et al, 2009; Pizzagalli et al, 2008; Tremblay et al, 2005) is a core symptoms in depression (Argyropoulos and Nutt, 1997; Rush and Weissenburger, 1994). Converging evidence from animal, pharmacological, and neuroimaging studies point toward NAcc dysfunction in depression and DBS of the NAcc leads to increases of all monoaminergic neurotransmitters in the PFC (van Dijk et al, 2012); this led to the hypothesis that DBS to the NAcc would lead to antidepressant effects by modulating the depression network (Schlaepfer et al, 2008).
Supero-lateral branch of the medial forebrain bundle
The supero-lateral branch of the medial forebrain bundle (slMFB) has also been proposed as a target (Coenen et al, 2011). Early lesional interventions have been found to exert their effect by influencing two major pathways (Schoene-Bake et al, 2010). These two affect-regulating fiber systems, the slMFB and the anterior thalamic radiation (ATR), are concerned with maintenance of emotional homeostasis. The slMFB is linked to reward-seeking and appetitive motivation (reward-seeking) in general, whereas the ATR is probably more involved in negative feelings (eg sadness, separation-distress, and psychic pain) (Coenen et al, 2012). Compared with neurological indications, higher stimulation intensities have been used in DBS for depression; the generated large electric fields thus stimulate structures beyond the intended target sites. Electric field simulation and probabilistic fiber tracking has demonstrated that the slMFB is anatomically and functionally connected with other DBS targets (Cg25, ALIC, and NAcc) (Coenen et al, 2012; Coenen et al, 2011). This lead to the hypothesis that most likely these targets are clinically effective because of a stimulation in a network that to a larger proportion is realized through the MFB a structure that had previously been identified to be involved in lesion surgery for depression (Schoene-Bake et al, 2010). A study using optogenetic neuromodulation together with DBS has recently shown that activation and modulation of afferent fiber tracts are a plausible mechanism of action in DBS (Gradinaru et al, 2009). Thus, modulation and not inactivation of the MFB would be postulated as the antidepressant mechanism of action (Coenen et al, 2012; Coenen et al, 2011). The VTA is an important relay station in the reward circuitry that serves a central role in motivation and reward processing (Lammel et al, 2014). This region projects via the MFB to the nucleus accumbens and via a separate pathway to the PFC. Very recently, two papers were published on optogenetic stimulation of the VTA. It was demonstrated that optogenetic recruitment of dopamine neurons potentially alters the neural encoding of depression-related behaviors in the downstream nucleus accumbens (Lammel et al, 2013; Tye et al, 2012). A second paper of different group demonstrated that optogenetic inhibition of the VTA–NAcc projection rapidly induced resilience, whereas inhibition of the VTA–mPFC projection promoted susceptibility in mice (Chaudhury et al, 2013). These results are insofar significant as it is likely that DBS to the MFB recruits the descending glutamatergic (and by that excitatory) projection (Russo and Nestler, 2013) from the PFC to the VTA (Schlaepfer et al, 2013). It needs to be considered that there are at least two distinct dopaminergic neuronal populations involved in the mechanisms of the VTA: (1) a population of tonic dopaminergic neurons that probably is related to reward promotion and (2) a population of phasic dopaminergic neurons which upon interference (or inhibition) result in increased resilience (Russo and Nestler, 2013). These distinct functions need to be further explored in future research but certainly have a role in the antidepressant effects of DBS to the MFB.
EFFICACY AND SAFETY
For three targets (Cg25, ALIC, and NAcc), acute and long-term antidepressant effects have been published (Bewernick et al, 2012; Bewernick et al, 2010; Holtzheimer et al, 2012c; Kennedy et al, 2011; Lozano et al, 2008; Malone et al, 2009; Puigdemont et al, 2011). In two studies, patients have been followed for up to 6 years (Bewernick et al, 2012; Kennedy et al, 2011). Sample sizes of these studies are small (<30) and sham control is not included in all studies (Blumberger et al, 2013). Thus, efficacy data are still on a pilot level (see Figure 1, Table 1; overview of published studies (Aouizerate et al, 2004; Bewernick et al, 2012; Bewernick et al, 2010; Dougherty et al, 2012; Grubert et al, 2011; Holtzheimer et al, 2012c; Jiménez et al, 2005; Kennedy et al, 2011; Lozano et al, 2012; Lozano et al, 2008; Malone et al, 2009; Mayberg et al, 2005; McNeely et al, 2008; Puigdemont et al, 2011; Sartorius et al, 2010; Schlaepfer et al, 2013; Schlaepfer et al, 2008)). A recent multicenter study on ALIC (Dougherty et al, 2012) has demonstrated the need for larger samples and raised a discussion on study design (see below).
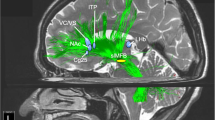
DBS targets in depression. Experimental targets (red spheres) for deep brain stimulation in major depression: (a) view from posterolateral right, (b) view from anterolateral right. Note how the medial forebrain bundle (green fiber structure) interconnects the majority of target sites (ALIC, NAcc, SCG, slMFB) as a key structure. Legend: ALIC, anterior limb of internal capsule; Hab, habenula; caudate, caudate nucleus; NAcc, nucleus accumbens (anatomically: accumbens); SCG, subgenual cingulate gyrus; slMFB, superolateral branch of the medial forebrain bundle.
Acute Effects
During surgery, intraoperative test stimulation is used to determine possible side effects and to assess acute clinical effects (Table 2). After surgery, at the initiation of chronic stimulation, repeated sessions within several weeks are required to determine best stimulation parameters (titration phase).
Immediate clinical effects, during intraoperative test stimulation or at the initiation of chronic stimulation occur within a few minutes. These effects include more spontaneous engagement in conversation, positive change in mood, increased alertness, relaxation, increased motivation, higher activity level, and a sense of calmness (Bewernick et al, 2010; Holtzheimer et al, 2012c; Mayberg et al, 2005), but also tension, dizziness, and anxiety. Only some patients experience acute effects, which do not seem predictive for long-term effects (Bewernick et al, 2010; Puigdemont et al, 2011). Possibly, an initial acute stimulation effect during surgery diminishes after re-initiation of stimulation (Holtzheimer et al, 2012c). In a recent pilot study of slMFB stimulation, all seven patients showed similar acute effects during intraoperative testing (eg, increased alertness, orientation reaction, spontaneous and appropriate engagement in conversation, eye contact), typically dominantly on one (but inter-individual distinct) side of intraoperative testing (Schlaepfer et al, 2013).
Longer-Term Clinical Effects
Long-term clinical effects are long-lasting changes that occur after 1–6 months of chronic DBS. Antidepressant response has been demonstrated in six small studies at three different targets (Cg25, ALIC, NAcc). (Bewernick et al, 2010; Kennedy et al, 2011; Lozano et al, 2012; Lozano et al, 2008; Malone et al, 2009; Puigdemont et al, 2011). Amelioration of other clinical measures (eg QoL, anxiety, general psychopathological burden) has been associated with antidepressant effects for these targets. When comparing outcomes of DBS studies, different ways of analyzing results has an impact on efficacy because most dropouts are non-responders. Therefore, intent-to-treat analysis (of all patients including dropouts up to the final endpoint, mostly in a carried forward manner) seems to be a more adequate, conservative method to reflect efficacy (see, for example, Bewernick et al, 2012; Puigdemont et al, 2011). Nonetheless, other groups present data analyzed as ‘per protocol/as treated’ (Holtzheimer et al, 2012c; Kennedy et al, 2011). In this way, dropouts and non-responders are not reflected in the data after their dropout, this leads to an artificially inflated efficacy. In addition, it is questionable whether a newly introduced time point ‘last observation’ is of scientific value, as it mostly reflects scores of patients that are treated for a short time and others treated long-term (up to many years) (see, for example, Malone, 2011). Response is uniformly described as a reduction of 50% or more reduction of the rating scale (Hamilton rating scale or depression or Montgomery Åsberg rating scale for depression) from baseline.
Response rates are similar for Cg25, ALIC, and NAcc (Bewernick et al, 2010; Lozano et al, 2008; Malone et al, 2009; Puigdemont et al, 2011). Twenty patients stimulated at Cg25 had a response rate of 55% after 1 year, 45% after 2 years, 60% after 3 years, and 55% response at the last follow-up visit (up to 6 years) in an intent-to-treat analysis (Kennedy et al, 2011). Similarly, a study with eight patients stimulating Cg24/25, reported response rates of 87% after 6 months and 62.5% after 12 months (n=8) (Puigdemont et al, 2011). In a mixed sample with 10 MDD patients and 7 patients suffering from bipolar disorder (Holtzheimer et al, 2012c), response rates for the patients still followed in the study (n=12 after 2 years) were 36% after 1 year (n=14), and 92% after 2 years (n=12) in a per protocol analysis. In a multicenter open-label trial targeting subgenual cingulate (n=21), 48% of patients responded after 6 months, and 29% after 12 months (Lozano et al, 2012) Seventeen patients were studied targeting the ALIC. Response rates were 53% after 12 months (n=17) and 71% at last follow-up (ranging from 14 to 67 months) (Malone et al, 2009).
Eleven patients were stimulated at the NAcc, 50% responded significantly during the first 6 months and remained stable during follow-up (up to 4 years), in an intent-to-treat analysis (Bewernick et al, 2012). Young, female patients with previous response to ECT and periods in remission after first onset of depression appear to benefit from DBS (Bewernick et al, 2010; Puigdemont et al, 2011) although small sample sizes limit the possibility to identify predictors of response. In addition, NAcc-DBS specifically influenced the symptoms of anhedonia and anxiety (Bewernick et al, 2010). Only recently, a hypothesis concerning exact electrode position has been assessed. In one study targeting Cg25, electrode position had an influence on antidepressant outcome (Puigdemont et al, 2011); among responders most patients had electrodes placed in Cg24. Another study did not find a relationship between electrode location and clinical effect (Lozano et al, 2008).
Pivotal study results in which DBS was not superior to sham stimulation, have been published from a randomized, sham-controlled, study of ALIC-DBS in 30 patients (Dougherty et al, 2012) contrasting results obtained at the same target (Malone et al, 2009). The percentage of patients responding to sham and active stimulation was similar (14.3% responding to sham, 20% to active stimulation) and the mean reduction in MADRS was larger in the sham stimulation group (−24.6%) compared with the real stimulation group (−19.6%). This study demonstrates how important design aspects in DBS studies are, here especially the amount of time used to identify optimum stimulation parameters and the point at which a sham condition was introduced in the study protocol (after stable antidepressant effects have been established or as staggered onset design, see below) are debatable.
A recent pilot study on DBS to the slMFB yielded interesting results: all patients showed strikingly similar intraoperative effects of increased appetitive motivation. Six of seven patients attained the response criterion; response was rapid—mean Montgomery-Åsberg Depression Rating Scale of the whole sample was reduced by >50% at day 7 after stimulation onset. At last observation (12–33 weeks), six patients were responders; among them, four were classified as remitters (Schlaepfer et al, 2013). In the only non-responder a hemorrhage occurred, and a tractographic post hoc analysis revealed that a significant amount of slMFB fibers that connect to the frontal lobe were missing due to the bleeding (Coenen et al, 2013). In the whole group, social functioning (Global Assessment of Functioning) improved from serious to mild impairment. Mean stimulation current was 2.86 mA. Currently a sham-controlled study with a staggered stimulation onset design is underway in order to further establish efficacy and safety at our center.
Putative Mode of Action of slMFB DBS
It is a little outside the scope of this review but the current interest in reward system DBS warrants a brief discussion on the topic: the mode of action of slMFB DBS at this moment remains unclear. There is circumstantial evidence, however, that allows for a plausible hypothesis and we have previously speculated about this (Schlaepfer et al, 2013). The slMFB contains short axons of unmyelinated dopaminergic neurons that cannot be directly recruited with DBS at short pulse widths (60 μs) (Ikemoto, 2010). The mode of action must be different and not through a direct activation. Glutamatergic fibers that descend from the PFC into the VTA have an activating effect. Very likely these heavily myelinated fibers are activated by the short pulse width (60 μs) used for slMFB DBS (Russo and Nestler, 2013). Most likely the DA neurons in the VTA are activated by these glutamatergic projections. Recently studies by Gale et al, in a primate model show that a chronic stimulation of the MFB in the zona incerta at high frequencies induces a DA release in the striatum (Gale et al, 2013). Also there is evidence from the OCD literature that DBS leads to a DA release (Figee et al, 2013). Obviously at least two DA-neuron populations are present in the VTA, tonic, and phasic neurons. Optogenetics teaches us that silencing highly active physic population increases resilience in mice. At the same time, activating the tonic DA neurons reduces susceptibility. We speculate that the tonic DA output from the VTA is increased and leads to increased free synaptic Dopamine in NAcc and the PFC. This is likely to increase appetitive motivation and might modulate reward expectancy. Uncovering the true effect of slMFB DBS is a very important next step and is the focus of our current research in animal models and human imaging studies.
Sham Stimulation Effects
Only few data on sham-controlled DBS in depression have been published hitherto (Bewernick et al, 2010; Holtzheimer et al, 2012c; Lozano et al, 2008) but small sample sizes do not allow the estimation of a sham effect. Significant sham effects in this group of very treatment-resistant patients seem improbable as likelihood of placebo response decreases with treatment resistance (Schatzberg and Kraemer, 2000). On the other hand, it has been shown in studies of DBS for Parkinson’s disorder that expectation and placebo effects account for clinically pertinent aspects of improvement with this procedure (Mercado et al, 2006). This together with the recent finding of high-sham response rates in ALIC-DBS for depression (Dougherty et al, 2012) really speaks for the need of large sham-controlled studies before DBS can be recommended clinically. Sham condition is difficult to maintain as have a hard time tolerating off phases leading to massive worsening of symptoms with increased risk for suicidal ideation. This problem could be partly addressed with staggered onset design protocols (Goodman et al, 2010), with a clear-cut rescue criterion for sham stimulation phases and weekly visits.
Cognition
Safety regarding cognitive effects has been documented for DBS to Cg25 (McNeely et al, 2008) and to NAcc (Grubert et al, 2011) and ALIC (Malone et al, 2009). Cognitive improvement in the domain of attention, memory, executive function, and visual perception has been demonstrated in patients treated with NAcc-DBS. This amelioration was not explained by the improvement in depression severity and could thus be shown independent of response status. There was a general trend toward cognitive normalization from below average, to average performance (Grubert et al, 2011). Until now, there is no evidence for cognitive enhancement effects above normal functioning at the evaluated target sites.
Adverse Effects
Side effects are related to the surgical procedure, to a malfunctioning of the DBS device or to the stimulation (see Table 1 for details). Wound infection after surgery or battery exchange, lead migration and device-related infections are important surgical complications in DBS studies. Lead migration (2.5% of patients), erosion, and infection (4.5–8.9% of patients) have been reported (Doshi, 2011; Fily et al, 2011). So far, there is only one report of hemorrhage in DBS studies for depression (Schlaepfer et al, 2013), but statistically, DBS surgery has a substantial of 0.9% to cause hemorrhage (Zrinzo et al, 2012). Side effects due to stimulation (eg erythema, increase in anxiety, agitation, and elevation of mood) are in most cases transient and occur within minutes to hours after new parameters have been programmed. The exact mechanism how side effects are induced is not fully understood, in some cases (eg oculomotor side effects), a modulation of neighboring neuronal tissue to the target region can explain the effect. If side effects persist and are judged to be troublesome, a change in stimulation settings is required. Careful assessment of patients is needed after parameters have been changed, especially if psychiatric side effects are possible.
The aggravation of symptoms due to battery depletion, unattended stimulation stop or during the blinding phase has been described (Bewernick et al, 2012; Holtzheimer et al, 2012b; Lozano et al, 2008). In spite of regular careful visits, suicides and suicide attempts have been reported (Bewernick et al, 2010; Holtzheimer et al, 2012b; Kennedy et al, 2011; Lozano et al, 2012). TRD is associated with a 15% risk of suicide (Isometsa et al, 1994; Wulsin et al, 1999). This risk is 4–5 times higher in severe depression compared with moderate or mild depression (Holtzheimer et al, 2012b). Thus, careful patient tracking is needed during follow-up; especially before optimal stimulation parameters have been established, after parameter change and during sham stimulation.
DISCUSSION
After a decade of DBS against depression, we are still away from effectively influencing dysfunctional emotional states. However, first studies have found encouraging antidepressant effects.
What Might be Truly Relevant Outcome Measures?
Traditional clinical rating scales
Depression studies commonly use depression scales (MADRS or HDRS); similar to pharmacotherapy studies, a 50% reduction in the measured depression score is judged as a significant response. This reduction reflects a major change in symptom load and in the patient’s QoL. It has been discussed, whether a reduction of cut-off for response to 40% is reasonable in DBS studies (Lozano et al, 2012), because many patients varied between 40% and 50% response during follow-up in this study. This already means for therapy-resistant patients a major change in QoL. Nonetheless, we believe that to maintain comparability with other therapies the conservative 50% criterion should be applied for efficacy evaluation. The commonly used depression rating scales however are not very sensitive in patients suffering from severe depression due to floor effects. Thus, new DBS-specific clinical measures are needed. Recently, a new putative measure, the Illness Density Index has been proposed, which might reflect DBS effects more adequately (Kelley et al, 2012).
Quality of life
In DBS studies for the treatment of neurological diseases, QoL has now been brought into focus, because a change in motor symptoms (eg in Parkinson’s disease), was not necessarily associated with an amelioration in QoL (Daniels et al, 2011). How important are QoL issues in DBS depression research? QoL scales measure dimensions beyond symptom improvement, eg abilities to interact socially, to enjoy leisure activities, to work effectively, and to manage everyday life. Treatment for MDD has been shown to improve QOL in the acute treatment phase, but QOL remains low compared with healthy controls even when symptoms are in remission following treatment (Ishak et al, 2011a; Ishak et al, 2011b; Kennedy et al, 2001). Changes of QoL seem to have different timelines as compared with symptom change (Kennedy et al, 2001). QoL is strongly related to the symptoms of depression (Daniels et al, 2011), but few studies exist exploring QoL in chronic, therapy-resistant depression (Miller et al, 1998). DBS studies assess changes in QoL using the medical outcomes study short-form SF-36 (Ware et al, 1998). Improvement in QoL in DBS depression studies have been reported for Cg25 (Kennedy et al, 2011) and NAcc (Bewernick et al, 2012), but the patients remained below average of healthy persons. Today, it is unclear, whether QoL changes in relation to response status. Thus, a QoL measure adds important information beyond symptom rating scales, especially when efficacy is not clear according to symptom rating scales.
OUTLOOK
After a decade of DBS for TRD, studies have shown relevant antidepressant effects. Nonetheless, with DBS is still associated with substantial surgical and psychiatric risks (eg hemorrhage, suicide) as well as high costs. Experience from first preliminary studies has lead to proposal of new target sites (Coenen et al, 2011; Mayberg et al, 2005) in a hypothesis-guided way. These new targets await rigorous scientific evaluation. Taken together, the data on DBS for major depression accumulated until today holds the promise that this intervention may lessen the suffering of those patients who hitherto have little or no hope to recover from treatment-resistant forms of the disease. This is remarkable. However, we have to remain modest and cognizant of the fact that DBS for neuropsychiatric disorders remains for now a ‘halfway technology’, a term created by Lewis Thomas to describe therapies that only ameliorate but not eliminate a disease condition (Olds and Milner, 1954). Thomas states that
“…It is characteristic of this kind of technology that it costs an enormous amount of money and requires a continuing expansion of hospital facilities. The only thing that can move medicine away from this level of technology is new information, and the only imaginable source of this information is research. The real high technology of medicine comes as the result of a genuine understanding of disease mechanisms.” (Thomas, 1971)
DBS certainly has the potential to be used as a powerful research tool, informing us about the underlying neurobiology of major depression and related psychiatric disorders. Already now it has contributed to a novel view of depression—moving away from a ‘synaptocentric’ view to a conceptualization of disordered brain networks, networks processing responses to affective stimuli (Krishnan and Nestler, 2008) including reward and reward anticipation (Russo and Nestler, 2013; Schlaepfer et al, 2013). It has become evident, that several psychiatric disorders might be correlated with network dysfunctions (Insel, 2010).
Research on DBS will most certainly lead to more effective treatments of depression, which might then in turn altogether use different forms of neuromodulation (Famm et al, 2013). Only when we fully understand the real underpinnings of major depression, a stimulation method can become a ‘decisive technology’ in Thomas’s terms and might even as a translational research strategy contribute to a new understanding of mental disorders. We believe that such a development is possible and that then DBS and its progressions into more refined neuromodulation strategies will deliver on today’s promises one day.
FUNDING AND DISCLOSURE
SK reports no biomedical financial interests or potential conflicts of interest. None of the authors is employed organization that may gain or lose financially through this publication. None of the authors owns stocks or shares in companies that may gain or lose financially through this publication; receives consultation fees or other forms of remuneration from organizations that may gain or lose financially or owns patents or patent applications whose value may be affected by publication. No funding specifically for conducting this review has been obtained.
References
Aouizerate B, Cuny E, Martin-Guehl C, Guehl D, Amieva H, Benazzouz A et al (2004). Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg 101: 574–575.
Argyropoulos SV, Nutt DJ (1997). Anhedonia and chronic mild stress model in depression. Psychopharmacology (Berl) 134: 333–336.
Baumeister AA (2000). The Tulane Electrical Brain Stimulation Program a historical case study in medical ethics. J History Neurosci 9: 262–278.
Berton O, Nestler EJ (2006). New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7: 137–151.
Bewernick B, Kayser S, Sturm V, Schlaepfer TE (2012). Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropschopharmacology 37: 1975–1985.
Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B et al (2010). Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry 67: 110–116.
Bishop MP, Elder ST, Heath RG (1963). Intracranial self-stimulation in man. Science 140: 394–396.
Blair-West GW, Cantor CH, Mellsop GW, Eyeson-Annan ML (1999). Lifetime suicide risk in major depression: sex and age determinants. J Affect Disord 55: 171–178.
Blumberger DM, Mulsant BH, Daskalakis ZJ (2013). What is the role of brain stimulation therapies in the treatment of depression? Curr Psychiatry Rep 15: 368.
Bourne SK, Eckhardt CA, Sheth SA, Eskandar EN (2012). Mechanisms of deep brain stimulation for obsessive compulsive disorder: effects upon cells and circuits. Front Integr Neurosci 6: 29.
Chang WS, Roh D, Kim CH, Chang JW (2013). Combined bilateral anterior cingulotomy and ventral capsule/ventral striatum deep brain stimulation for refractory obsessive-compulsive disorder with major depression: do combined procedures have a long-term benefit? Restor Neurol Neurosci 31: 723–732.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW et al (2013). Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493: 532–536.
Coenen V, Honey C (2009a). Ablative procedures for depression. In: Lozano A, Gildenberg PL, Tasker RR (eds). Textbook of Stereotatcic and Functional Neurosurgery. Springer: Berlin, Heidelberg. pp 2943–2952.
Coenen VA, Honey CR, Hurwitz T, Rahman AA, McMaster J, Burgel U et al (2009b). Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson’s disease. Neurosurgery 64: 1106–1114.
Coenen VA, Mädler B, Schlaepfer TE (2013). Reply to: medial forebrain bundle stimulation-speed access to an old or entry into a new depression neurocircuit? Biol Psychiatry 74: e45–e46.
Coenen VA, Panksepp J, Hurwitz TA, Urbach H, Madler B (2012). Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J Neuropsychiatry Clin Neurosci 24: 223–236.
Coenen VA, Schlaepfer TE, Maedler B, Panksepp J (2011). Cross-species affective functions of the medial forebrain bundle-implications for the treatment of affective pain and depression in humans. Neurosci Biobehav Rev 35: 1971–1981.
Crupi R, Marino A, Cuzzocrea S (2011). New therapeutic strategy for mood disorders. Curr Med Chem 18: 4284–4298.
Daniels C, Krack P, Volkmann J, Raethjen J, Pinsker MO, Kloss M et al (2011). Is improvement in the quality of life after subthalamic nucleus stimulation in Parkinson’s disease predictable? Mov Disord 26: 2516–2521.
Delgado JMR (1971) Physical Control of the Mind — Toward a Psychocivilized Society. Harper Colophon books: New York.
Doshi PK (2011). Long-term surgical and hardware-related complications of deep brain stimulation. Stereotact Funct Neurosurg 89: 89–95.
Dougherty D, Carpenter L, Bhati M, Howland R, O’Reardon J, Denko T et al (2012). A Randomized Sham-Controlled Trial of DBS of the VC/VS for Treatment-Resistant Depression. Society of Biological Psychiatry 67th Annual Scientific Convention. 071-Late Breaking Oral Session #2—Mixed Topics http://goo.gl/PGTpo.
Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M (2013). Drug discovery: a jump-start for electroceuticals. Nature 496: 159–161.
Farah MJ, Illes J, Cook-Deegan R, Gardner H, Kandel E, King P et al (2004). Neurocognitive enhancement: what can we do and what should we do? Nat Rev Neurosci 5: 421–425.
Figee M, de Koning P, Klaassen S, Vulink N, Mantione M, van den Munckhof P et al (2013). Deep brain stimulation induces striatal dopamine release in obsessive-compulsive disorder. Biol Psychiatry S0006-3223: 00631–00638.
Fily F, Haegelen C, Tattevin P, Buffet-Bataillon S, Revest M, Cady A et al (2011). Deep brain stimulation hardware-related infections: a report of 12 cases and review of the literature. Clin Infect Dis 52: 1020–1023.
Gale JT, Lee KH, Amirnovin R, Roberts DW, Williams ZM, Blaha CD et al (2013). Electrical stimulation-evoked dopamine release in the primate striatum. Stereotact Funct Neurosurg 91: 355–363.
Goodman WK, Foote KD, Greenberg BD, Ricciuti N, Bauer R, Ward H et al (2010). Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry 67: 535–542.
Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K (2009). Optical deconstruction of parkinsonian neural circuitry. Science 324: 354–359.
Greenberg BD, Askland KD, Carpenter LL (2008). The evolution of deep brain stimulation for neuropsychiatric disorders. Front Biosci 13: 4638–4648.
Grubert C, Hurlemann R, Bewernick BH, Kayser S, Hadrysiewicz B, Axmacher N et al (2011). Neuropsychological safety of nucleus accumbens deep brain stimulation for major depression: effects of 12-month stimulation. World J Biol Psychiatry 12: 516–527.
Hariz MI, Blomstedt P, Zrinzo L (2010). Deep brain stimulation between 1947 and 1987: the untold story. Neurosurg Focus 29: E1.
Heath RG (1954) Studies In Schizophrenia. Harvard University Press: Cambridge, MA. pp 46–47.
Holtzheimer PE 3rd, Kosel M, Schlaepfer T (2012a). Brain stimulation therapies for neuropsychiatric disease. Handb Clin Neurol 106: 681–695.
Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A et al (2012b). Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry 69: 150–158.
Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A et al (2012c). Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry 69: 150–158.
Howe MW, Tierney PL, Sandberg SG, Phillips PE, Graybiel AM (2013). Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature 500: 575–579.
Hurwitz TA, Honey CR, Allen J, Gosselin C, Hewko R, Martzke J et al (2012). Bilateral anterior capsulotomy for intractable depression. J Neuropsychiatry Clin Neurosci 24: 176–182.
Ikemoto S (2010). Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev 35: 129–150.
Insel TR (2010). Faulty circuits. Scientific American 302: 44–51.
Ishak WW, Greenberg JM, Balayan K, Kapitanski N, Jeffrey J, Fathy H et al (2011a). Quality of life: the ultimate outcome measure of interventions in major depressive disorder. Harv Rev Psychiatry 19: 229–239.
Ishak WW, Ha K, Kapitanski N, Bagot K, Fathy H, Swanson B et al (2011b). The impact of psychotherapy, pharmacotherapy, and their combination on quality of life in depression. Harv Rev Psychiatry 19: 277–289.
Isometsa ET, Henriksson MM, Aro HM, Heikkinen ME, Kuoppasalmi KI, Lonnqvist JK (1994). Suicide in major depression. Am J Psychiatry 151: 530–536.
Jiménez F, Velasco F, Salin-Pascual R, Hernández JA, Velasco M, Criales JL et al (2005). A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. J Neurosurg 57: 585–593.
Kelley ME, Franco AR, Mayberg HS, Holtzheimer PE (2012). The Illness Density Index (IDI): a longitudinal measure of treatment efficacy. Clin Trials 9: 596–604.
Kennedy SH, Eisfeld BS, Cooke RG (2001). Quality of life: an important dimension in assessing the treatment of depression? J Psychiatry Neurosci 26 Suppl: S23–S28.
Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS et al (2011). Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry 168: 502–510.
Krishnan V, Nestler EJ (2008). The molecular neurobiology of depression. Nature 455: 894–902.
Lammel S, Lim BK, Malenka RC (2014). Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76 Pt B: 351–359.
Lammel S, Tye KM, Warden MR (2013). Progress in understanding mood disorders: optogenetic dissection of neural circuits. Genes Brain Behav 13: 38–51.
Lippitz BE, Mindus P, Meyerson BA, Kihlstrom L, Lindquist C (1999). Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive-compulsive disorder: relevance of the right hemisphere. Neurosurgery 44: 452–458.
Lipsman N, Neimat JS, Lozano AM (2007). Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: the search for a valid target. Neurosurgery 61: 1–11.
Lisanby SH (2007). Electroconvulsive therapy for depression. N Engl J Med 357: 1939–1945.
Lozano AM, Giacobbe P, Hamani C, Rizvi SJ, Kennedy SH, Kolivakis TT et al (2012). A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg 116: 315–322.
Lozano AM, Lipsman N (2013). Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 77: 406–424.
Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH (2008). Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry 64: 461–467.
Malone DA (2011). Use of deep brain stimulation in treatment-resistant depression. Cleve Clin J Med 77: 77.
Malone DA Jr., Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN et al (2009). Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry 65: 267–275.
Mayberg HS (1997). Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 9: 471–481.
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C et al (2005). Deep brain stimulation for treatment-resistant depression. Neuron 45: 651–660.
McNeely HE, Mayberg HS, Lozano AM, Kennedy SH (2008). Neuropsychological impact of Cg25 deep brain stimulation for treatment-resistant depression: preliminary results over 12 months. J Nerv Ment Dis 196: 405–410.
Mercado R, Constantoyannis C, Mandat T, Kumar A, Schulzer M, Stoessl AJ et al (2006). Expectation and the placebo effect in Parkinson’s disease patients with subthalamic nucleus deep brain stimulation. Mov Disord 21: 1457–1461.
Meyer A, Beck E, Mc LT (1947). Prefrontal leucotomy; a neuro-anatomical report. Brain 70: 18–49.
Miller IW, Keitner GI, Schatzberg AF, Klein DN, Thase ME, Rush AJ et al (1998). The treatment of chronic depression, part 3: psychosocial functioning before and after treatment with sertraline or imipramine. J Clin Psychiatry 59: 608–619.
Miocinovic S, Somayajula S, Chitnis S, Vitek JL (2013). History, applications, and mechanisms of deep brain stimulation. JAMA Neurol 70: 163–171.
Moniz E (1994). Prefrontal leucotomy in the treatment of mental disorders. 1937. Am J Psychiatry 151 (Suppl 6): 236–239.
Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B (1999). Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet 354: 1526.
Olds J, Milner P (1954). Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 47: 419–427.
Papez JW (1995). A proposed mechanism of emotion. 1937. J Neuropsychiatry Clin Neurosci 7: 103–112.
Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R et al (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 166: 702–710.
Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M (2008). Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res 43: 76–87.
Puigdemont D, Perez-Egea R, Portella MJ, Molet J, de Diego-Adelino J, Gironell A et al (2011). Deep brain stimulation of the subcallosal cingulate gyrus: further evidence in treatment-resistant major depression. Int J Neuropsychopharmacol 15: 121–133.
Riva-Posse P, Holtzheimer PE, Garlow SJ, Mayberg HS (2013). Practical considerations in the development and refinement of subcallosal cingulate white matter deep brain stimulation for treatment-resistant depression. World Neurosurg 80: S27 e25–S27.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D et al (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163: 1905–1917.
Rush AJ, Weissenburger JE (1994). Melancholic symptom features and DSM-IV. Am J Psychiatry 151: 489–498.
Russo SJ, Nestler EJ (2013). The brain reward circuitry in mood disorders. Nat Rev Neurosci 14: 609–625.
Sartorius A, Henn FA (2007). Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses 69: 1305–1308.
Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW et al (2010). Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry 67: e9–e11.
Schatzberg AF, Kraemer HC (2000). Use of placebo control groups in evaluating efficacy of treatment of unipolar major depression. Biol Psychiatry 47: 736–744.
Schlaepfer TE, Bewernick BH, Kayser S, Madler B, Coenen VA (2013). Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry 73: 1204–1212.
Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N et al (2008). Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33: 368–377.
Schlaepfer TE, George MS, Mayberg H (2010). WFSBP Guidelines on brain stimulation treatments in psychiatry. World J Biol Psychiatry 11: 2–18.
Schoene-Bake JC, Parpaley Y, Weber B, Panksepp J, Hurwitz TA, Coenen VA (2010). Tractographic analysis of historical lesion surgery for depression. Neuropsychopharmacology 35: 2553–2563.
Shorter E, Healy D (2007) Shock Therapy: A History of Electroconvulsive Treatment in Mental Illness. University of Toronto Press: Toronto.
Spiegel EA, Wycis HT, Marks M, Lee AJ (1947). Stereotaxic apparatus for operations on the human brain. Science 106: 349–350.
Thomas L (1971). The technology of medicine. N Engl J Med 285: 1366–1368.
Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S et al (2005). Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry 62: 1228–1236.
Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J et al (2012). Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493: 537–541.
van Dijk A, Klompmakers AA, Feenstra MG, Denys D (2012). Deep brain stimulation of the accumbens increases dopamine, serotonin and noradrenaline in the prefrontal cortex. J Neurochem 123: 897–903.
Ware JE Jr., Kosinski M, Gandek B, Aaronson NK, Apolone G, Bech P et al (1998). The factor structure of the SF-36 Health Survey in 10 countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 51: 1159–1165.
Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE et al (2013). Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382: 1575–1586.
Wulsin LR, Vaillant GE, Wells VE (1999). A systematic review of the mortality of depression. Psychosom Med 61: 6–17.
Zrinzo L, Foltynie T, Limousin P, Hariz MI (2012). Reducing hemorrhagic complications in functional neurosurgery: a large case series and systematic literature review. J Neurosurg 116: 84–94.
Acknowledgements
TES and VAC received partial funding for two investigator-initiated studies on DBS for major depression from Medtronic Inc. and the Hope for Depression Research Foundation (HDRF) and the Institute for Affective Neuroscience (ISAN). VAC is consultant for Medtronic Europe and occasionally received honoraria for talks. TS is chair of the project group, ‘Deep Brain Stimulation in Psychiatry: Guidance for Responsible Research and Application’, funded by the Volkswagen Foundation (Hanover, Germany). TS and BB are members of the working Group Neuromodulation of the German Research Foundation. RH was supported by a Starting Independent Researcher Grant (NEMO—Neuromodulation of Emotion) jointly provided by the Ministry of Innovation, Science, Research and Technology of the German State of North Rhine-Westphalia (MIWFT) and the University of Bonn.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Schlaepfer, T., Bewernick, B., Kayser, S. et al. Deep Brain Stimulation of the Human Reward System for Major Depression—Rationale, Outcomes and Outlook. Neuropsychopharmacol 39, 1303–1314 (2014). https://doi.org/10.1038/npp.2014.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.28
Keywords
This article is cited by
-
Accelerated TMS - moving quickly into the future of depression treatment
Neuropsychopharmacology (2024)
-
Normalized affective responsiveness following deep brain stimulation of the medial forebrain bundle in depression
Translational Psychiatry (2024)
-
Linking connectivity of deep brain stimulation of nucleus accumbens area with clinical depression improvements: a retrospective longitudinal case series
European Archives of Psychiatry and Clinical Neuroscience (2024)
-
Deep brain stimulation of the “medial forebrain bundle”: a strategy to modulate the reward system and manage treatment-resistant depression
Molecular Psychiatry (2022)
-
Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications
Molecular Psychiatry (2022)