Abstract
The neuropeptide oxytocin has recently been shown to enhance eye gaze and emotion recognition in healthy men. Here, we report a randomized double-blind, placebo-controlled trial that examined the neural and behavioral effects of a single dose of intranasal oxytocin on emotion recognition in individuals with Asperger syndrome (AS), a clinical condition characterized by impaired eye gaze and facial emotion recognition. Using functional magnetic resonance imaging, we examined whether oxytocin would enhance emotion recognition from facial sections of the eye vs the mouth region and modulate regional activity in brain areas associated with face perception in both adults with AS, and a neurotypical control group. Intranasal administration of the neuropeptide oxytocin improved performance in a facial emotion recognition task in individuals with AS. This was linked to increased left amygdala reactivity in response to facial stimuli and increased activity in the neural network involved in social cognition. Our data suggest that the amygdala, together with functionally associated cortical areas mediate the positive effect of oxytocin on social cognitive functioning in AS.
Similar content being viewed by others
INTRODUCTION
The ability to infer socially relevant information such as emotions from facial expressions is fundamental to human social interaction. Impairments in the processing of faces, such as reduced attention to faces (Chawarska et al, 2010), diminished eye gaze (Klin et al, 2002), and deficits in recognition of emotions (Dawson et al, 2005; Harms et al, 2010) and other social information from faces (Adolphs et al, 2001) constitute typical social deficits in individuals with autism spectrum disorders (ASD). ASD is a cluster of pervasive developmental disorders comprising autism, Asperger’s disorder (or Asperger syndrome; AS), and pervasive developmental disorder not otherwise specified. Functional imaging studies have provided evidence for alterations in the neural circuitry involved in the processing of social information in ASD, including in the amygdala (Dalton et al, 2005), the fusiform gyrus (Schultz et al, 2003), and the posterior superior temporal lobe (Pelphrey et al, 2005). Recently, it has been proposed that alterations in the central nervous oxytocin system might be associated with the social impairments characteristic of individuals with ASD (Carter, 2007).
Oxytocin is a nine amino-acid neuropeptide that is fundamentally involved in the social behaviors of both rodents (Donaldson and Young, 2008) and humans (Meyer-Lindenberg et al, 2011). In healthy adults, oxytocin has been shown to reduce anxiety and endocrine responses to social stress (Chen et al, 2011; Heinrichs et al, 2003), to promote trusting behavior (Kosfeld et al, 2005), and to improve social memory (Guastella et al, 2008b; Rimmele et al, 2009). In regards to face processing, oxytocin has been shown to improve emotion recognition (Lischke et al, 2012a; Marsh et al, 2010) and the ability to infer the mental and emotional states of others from subtle facial cues (Domes et al, 2007b; Domes et al, 2012; Schulze et al, 2011), and to increase eye gaze to neutral and emotional human faces (Domes et al, 2013b; Gamer et al, 2010; Guastella et al, 2008a). On the neural level, recent electrophysiological research in animals (Viviani et al, 2011) and functional neuroimaging studies in humans (Baumgartner et al, 2008; Domes et al, 2007a; Domes et al, 2010; Gamer et al, 2010; Kirsch et al, 2005; Lischke et al, 2012b) suggest that the amygdala is a central region involved in the behavioral effects of oxytocin. In addition, whole brain analyses have revealed modulatory effects of oxytocin in prefrontal and temporal areas as well as in the brainstem (Zink and Meyer-Lindenberg, 2012).
Some genetic association studies suggest the involvement of structural variations of the oxytocin receptor gene in the development of ASD (Jacob et al, 2007; Lerer et al, 2007; Liu et al, 2010; Wermter et al, 2010; Wu et al, 2005), although the evidence is not consistent and a recent meta-analysis did not find an overall effect for the two most frequently investigated single-nucleotide polymorphisms (rs53576 and rs2254298) in ASD (Bakermans-Kranenburg and van Ijzendoorn, 2013). Experimental studies have provided evidence that the administration of oxytocin might improve affective speech comprehension (Hollander et al, 2007), enhance emotion recognition, (Guastella et al, 2009) increase eye gaze and promote social interaction (Andari et al, 2010) in individuals with ASD, and increase responding of the amygdala to faces in an identity matching task (Domes et al, 2013a).
On the basis of the results reported in neurotypical individuals and the initial findings on the beneficial effects of oxytocin on social cognition in ASD, we hypothesized that individuals with ASD would show increased emotion recognition performance following a single dose of intranasally administered oxytocin. Furthermore, we expected increased activity in the neural circuitry involved in facial emotion recognition following oxytocin treatment, in particular in the amygdala, the fusiform gyrus, and the superior temporal lobe. Given that individuals with ASD tend to disregard the relevance of the eye region in social communication (Klin et al, 2002), and are impaired in reading the ‘language of the eyes’ (Baron-Cohen et al, 2001), we expected that the effects of oxytocin would be pronounced for this particular social stimulus. To test these predictions, we conducted an experiment comparing the effects of oxytocin on emotion recognition from the eye as opposed to the mouth region while regional brain activity was measured with a magnetic resonance imaging (MRI) scanner.
MATERIALS AND METHODS
Participants
Participants were 14 adult males with Asperger’s Disorder (or Asperger syndrome; AS) according to the DSM-IV and 14 healthy, typically developing, age-matched male controls (CON) (mean age+/−SD; AS: 24.0+/−6.9 years; CON: 23.6+/−5.4 years; t(26)=0.17; P=0.87). Participants with AS were diagnosed by a trained and clinically experienced psychiatrist with the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al, 1994), and Module 4 of the Autism Diagnostic Observation Schedule (ADOS-R) (Lord et al, 2000). All participants with AS met the cutoff score for Autistic Disorder in the ADOS-R. Using the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) as an estimate of general intelligence, we did not find group differences in intellectual abilities (mean IQ+/−SD AS: 122.4+/−24.1; CON: 125.6+/−15.4; t(26)=0.42; P=0.60). Additional information on demographic group characteristics is provided in Supplementary Table S1. All participants were not taking medication, had normal or corrected-to-normal vision, and reported no history of neurological, psychiatric (other than autism), or endocrine disorders. The present data were collected as part of a larger project on the neural effects of oxytocin in ASD. Data on face discrimination from the same project have been published previously (Domes et al, 2013a).
Procedure
Before the experimental sessions, all participants gave written informed consent and were screened for the presence of neurological, psychiatric, and somatic illness. Thereafter, participants completed a set of questionnaires (see Supplementary Methods) and were familiarized with the administration of the nasal spray, the imaging procedures, and the experimental procedure during scanning. Participants were instructed to abstain from smoking, caffeine, and analgesic medication on the scanning days.
Two experimental sessions were conducted in a randomized double-blind, placebo-controlled, within-subject, cross-over design. Forty-five minutes before the functional imaging sessions, participants self-administered three puffs per nostril of oxytocin (Syntocinon-Spray, Novartis, Switzerland; each puff with 4 IU oxytocin; total dose of 24 IU oxytocin) or placebo (containing all ingredients except for the peptide) under the supervision of the experimenter. To assess possible oxytocin-induced changes in mood, wakefulness, and calmness, participants completed a three-scale state questionnaire before substance administration and before entering the scanner (Steyer et al, 1997). The experiment lasted 3 h (45 min total scanning time). Participants received monetary compensation after completion of the study. All procedures were in accordance with the Declaration of Helsinki and were approved by the local ethics committee.
Facial Emotion Recognition Task
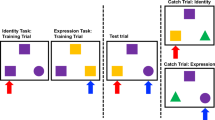
Black-and-white photographs of four different individuals (two male, two female) displaying six basic facial emotions (anger, fear, disgust, happiness, sadness, and surprise) were taken from a standardized set of faces (Eckman and Friesen, 1976). Sections of the eye region and the mouth region were extracted from each picture. The resulting 48 pictures (24 eyes and 24 mouths) had a size of 390 × 150 pixels and were presented for 3 s at the center of a 800 × 600 pixel light grey screen. Facial stimuli were immediately followed by an emotional verbal label presented for 3 s. Half of the stimuli were presented followed by the correct label and half followed by an incorrect label. On each trial, participants were asked to indicate whether the label was correct or incorrect by pressing a button. Trials were presented with an inter-stimulus interval of 1.7 s. (trial structure—Figure 1a). In order to provide a baseline condition and to enhance design efficiency, 24 rest trials (a grey box comparable in luminescence and size to the facial sections) were presented randomly. The total time of the experiment was 9:40 min. Facial stimuli were presented with a pair of MRI-compatible LCD goggles (VisuaStim Digital, Resonance Technology, Northridge CA, USA) using Presentation 14.0 (Neurobehavioral Systems, Albany, CA, USA) allowing for response accuracy and latencies. Emotion recognition performance was analyzed based on two-high-threshold model discrimination indices pr (Snodgrass and Corwin, 1988) for the eye and mouth condition by subtracting false alarm rates (FA) from hit rates (H): Pr=H−FA. Effects of oxytocin on response bias (ie, more or less liberal responding to targets in general) were assessed by calculating Br: Br=FA/(1—(H−FA)). A liberal bias results in Br>0.5 and a conservative bias is indicated by Br<0.5 (Snodgrass and Corwin, 1988).
Experimental design and emotion recognition performance. (a) Trial structure and experimental conditions. (b) Effects of oxytocin on emotion recognition (discrimination index Pr) in individuals with Asperger syndrome (AS) and NT controls as a function of stimulus type (eyes vs mouth). In individuals with AS, oxytocin administration increased emotion recognition from the eye region, whereas this was not the case in NT controls.
Magnetic Resonance Imaging
Functional and structural images were acquired with a 1.5 Tesla Scanner (Siemens Avanto) equipped with high-speed gradients and the 12-channel standard Head Matrix Coil (Siemens, Erlangen, Germany). In order to reduce artifacts due to head motion, a vacuum cushion was used to stabilize the head, and participants were instructed to stay still during scanning. Following an anatomical scout for slice positioning, 214 volumes containing 36 interleaved axial slices (3 mm thickness with 1 mm gap) covering the whole brain were acquired using a T2*-sensitive echo-planar-imaging (EPI) sequence (Echo time (TE)=40 ms, Repetition time (TR)=2700 ms, Flip-angle=90°, Field of View (FoV)=214 × 214 mm, Matrix=64 × 64). Following the functional scans, a structural image was acquired with a three-dimensional, T1-weighted, gradient-echo (MPRAGE) sequence (160 sagital slices, 1 mm slice thickness, TE=3.9 ms, TR=1500 ms, Flip-angle=15°, FoV=256 × 256 mm, Matrix=256 × 256).
Image Preprocessing and Statistical Analyses
Preprocessing and statistical analyses were performed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm). The first five volumes of each functional series were discarded to reduce T1 saturation artifacts. Preprocessing included realignment of the images to the first image in the series, coregistration of the functional images and the individual anatomical image, segmentation, spatial normalization to the Montreal Neurological Institute (MNI) standard brain, and spatial smoothing (Gaussian kernel of 9 mm FWHM).
On the first-level, data for the two scanning sessions were compiled and treated as separate runs for each individual. We then modeled each event (eye vs mouth) of the two runs of a specific condition as box-car functions of 3 s duration starting with the onset of picture presentation convolved with a hemodynamic response function. In order to reduce slow drift artifacts, a high-pass filter with a cutoff period of 128 s was applied to the voxel time courses. Fixed-effects analysis was conducted by estimating regression coefficients for each condition using least squares within SPM8. Contrast estimates were then calculated for the following differential contrasts: eyes (oxytocin>placebo), mouth (oxytocin>placebo), and the reverse contrasts.
For second-level random-effects analyses, first-level contrasts were subject to a two-sample t-test, testing for oxytocin-induced effects during emotion recognition from the eyes and mouth stimuli within and between both groups. Region-of-interest (ROI) analysis for the amygdala was conducted using a sphere ROI of 5 mm radius around the peak voxel within the anatomical amygdala templates provided by the AAL database (Tzourio-Mazoyer et al, 2002). A small volume correction (SVC) for the statistical threshold of P<0.05 was applied for ROI analysis. Percent signal change (PSC) was extracted using rfxplot (Glascher, 2009) for the averaged signal from these ROI, and plotted to illustrate BOLD signal changes in response to facial stimuli in both drug conditions. Exploratory whole brain analysis was then conducted using an uncorrected statistical threshold of P<0.001 with a cluster extent threshold of k⩾10. Coordinates of peak voxels within significant clusters are given in MNI space (Evans et al, 1993).
RESULTS
Effects of OT on Emotion Recognition
Participants with AS showed impaired overall emotion recognition performance compared with NT controls (main effect of group: F1,26=20.68; P<0.001), and emotions were easier to recognize from the eye compared with the mouth stimuli (main effect of stimulus category: F1,26=39.94; P<0.001). In the placebo condition, individuals with AS showed reduced recognition performance for eyes (t26=3.70; P<0.01) and for the mouth (t26=3.59; P<0.01) stimuli compared with NT controls. There was a trend for oxytocin to improve emotion recognition performance (main effect of drug condition: F1,26=3.57; P=0.07), and a significant drug-by-stimulus interaction, reflecting stronger (and more consistent) effects of oxytocin for eyes compared with the mouth stimuli (F1,26=5.95; P<0.05). In addition, there was a significant drug-by-group interaction, suggesting stronger effects of oxytocin for participants with AS compared with NT controls (F1,26=4.56; P<0.05). In order to specify this interaction, separate 2-way ANOVAs for both groups were calculated: in participants with AS, oxytocin administration improved emotion recognition regardless of stimulus category (main effect of drug condition: F1,13=4.75; P<0.05; drug × stimulus interaction: F1,13=2.50; P=0.138). In contrast, NT controls showed no overall improvement of emotion recognition following oxytocin administration (main effect of drug condition: F1,13=0.10; P=0.834). Notably, the only significant pair-wise comparison regarding the effect of oxytocin was found for emotion recognition from the eyes in participants with AS (t13=2.32; P<0.05). (Figure 1b). Additional analyses on hit rates, false alarms rates, and response biases can be found in Table 1. To test for effects of oxytocin on wakefulness, calmness, and mood separate 3-way ANOVAs (group × drug × time) were calculated. There were no significant main effects of the drug condition, and no interaction effects involving the drug factor (all P>0.10; Supplementary Table S2).
ROI Analyses: OT Effects on Amygdala Reactivity
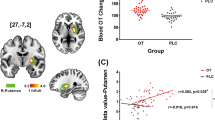
In participants with AS, analysis for amygdala activity showed increased responding of the left amygdala to both eye stimuli (x, y, and z: −18, −1, −23; t=3.60, k=11; P(SVC)=0.007), as well as mouth stimuli (x, y, and z: −18,−1, −23; t=4.39, k=11; P(SVC)=0.0009) following oxytocin treatment compared with placebo (contrast: ASOXT>PLA). Similar effects were not observed in NT controls: there was neither an effect of oxytocin in the anatomical amygdala template during emotion recognition from the eyes nor from the mouth (Figure 2). In addition, no such effects were observed in the right amygdala or for the reverse contrast on both sides.
Effects of oxytocin on amygdala activity in individuals with Asperger syndrome (AS) and NT controls as a function of stimulus type (eyes vs mouth). (a) Oxytocin significantly increased the activation of the left amygdala (region-of-interest, ROI) in individuals with AS while viewing eyes (x, y, z: −18, −1, −23; t=3.60, k=11; P(small volume correction, SVC)=0.007) and mouth stimuli (x, y, z: −18, −1,−23; t=4.39, k=11; P(SVC)=0.0009). Statistical parametric maps are displayed with a threshold of p(uncorr)<0.005; k=10. (b) Percent signal change in the amygdala ROI (27 voxels) as a function of group, stimulus type, and drug condition. For individuals with AS, effects of oxytocin were comparable for eyes and mouth stimuli, whereas no such effect was observed in NT controls.
In order to test for linear associations between oxytocin-induced modulation of amygdala reactivity and emotion recognition performance, Pearson’s correlations between the increase in PSC in the left amygdala (oxytocin—placebo) and the improvement in emotion recognition performance (averaged Pr for eyes and mouth stimuli) were calculated. In the AS group, we found a significant correlation (r=0.47; P<0.05, single-sided) between oxytocin-induced increase in amygdala reactivity and emotion recognition (averaged for the eye and mouth stimuli), this was not the case in the NT control group (r=0.14; P=0.63) Figure 3.
Linear association between oxytocin-induced modulation of left amygdala reactivity to facial stimuli and emotion recognition performance. In the Asperger syndrome (AS group), oxytocin-induced increase in amygdala reactivity predicted the improvement in emotion recognition (r=0.47; P<0.05, single-sided), whereas this was not the case in the NT control group (r=0.14; P=0.63).
Effects of Drug Sequence
Sequence effects of drug administration were tested for both emotion recognition performance and functional brain imaging. Including sequence of drug conditions as an additional factor in a four-way ANOVA did not reveal additional significant effects, indicating that the observed effects of oxytocin on emotion recognition performance were independent of whether oxytocin was given at the first scan or at the second.
In order to test for sequence effects of drug conditions on amygdala activity, we extracted PSC data from the left amygdala ROI and calculated a four-way ANOVA with the following factors: group, stimulus category, drug condition, and sequence of drug conditions. Except for the main effect of drug (F(1,24)=8.14; P=0.009), the drug × group (F(1,24)=4.68; P=0.041), and the drug × stimulus category interaction (F(1,24)=4.75; P=0.039), no other effect reached significance, indicating that the observed effect of oxytocin was independent of whether oxytocin was administered at the first scan or at the second.
Whole Brain Analysis
Whole brain analysis was conducted to explore oxytocin-induced modulations of regional brain activity during facial emotion recognition beyond the predefined ROI in the amygdala. In participants with AS, we found significantly increased activation in the temporal pole, superior temporal cortex, inferior frontal gyrus, Supplementary Motor Area, cerebellum, and superior parietal lobe following oxytocin administration compared with placebo (contrast: ASOXT>PLA) for both eye stimuli and mouth stimuli. Using the same combined statistical and extend threshold (puncorrected<0.001 and k>10) there were only two significant clusters in NT controls showing increased activation following oxytocin administration compared with placebo (contrast: NTOXT>PLA): the inferior frontal gyrus (for eye stimuli) and the fusiform gyrus (for mouth stimuli). Contrasting these effects explicitly (contrast: ASOXT>PLA>NTOXT>PLA) revealed only two clusters: one in the ventro-lateral prefrontal cortex and one in the cerebellum. In both groups, the reverse contrasts (ASPLA>OXT and NTPLA>OXT) did not reveal significant clusters, indicating that regional brain activity in response to facial stimuli was not attenuated under oxytocin compared with placebo. A complete list of activated clusters is given in Table 2.
DISCUSSION
In accordance with our hypothesis, and in line with previous research in adolescents with ASD (Guastella et al, 2009), a single dose of intranasally administered oxytocin improved performance on a basic emotion recognition task in adults with AS, and to a lesser extent in neurotypical controls. This effect was more pronounced for the inference of emotions from the eye region compared with the mouth region, and could not be explained by enhanced response latencies or a drug-induced response bias toward more liberal answering.
Autistic individuals often disregard the relevance of social information from the eye region (Klin et al, 2002) and show difficulties in interpreting social information from the eyes (Baron-Cohen et al, 2001). Improved emotion recognition from the eye region found in the present study is consistent with recent studies showing enhanced gaze to the eye region after intranasal oxytocin administration in healthy volunteers (Andari et al, 2010; Domes et al, 2013b; Gamer et al, 2010). Taken together, these studies suggest that oxytocin-induced enhancement of attention to the eye region (Andari et al, 2010) might be the mediating factor for the beneficial effect on emotion recognition in autistic individuals.
At the neural level, oxytocin administration specifically increased amygdala reactivity to facial stimuli in the AS group. Participants with AS showed increased activations in the left amygdala, regardless of whether eye or mouth stimuli were shown. No such effect was observed in NT controls. The implication of the amygdala in mediating emotion recognition performance is supported by the positive correlation between increased amygdala activation and emotion recognition performance in the AS group observed in the present study. Numerous studies have provided evidence that amygdala activity is associated with fear and emotion processing, face perception, affective learning, and more broadly with the motivational relevance and salience of the stimuli presented (for a recent review: Adolphs, 2010). Thus, increased responding of the amygdala following oxytocin administration in the present study might reflect increased allocation of cognitive resources to the presented stimuli, and might therefore represent part of the neural basis for increased emotion recognition performance. The evidence regarding amygdala activity during face perception in adults with ASD is inconsistent, and includes studies both showing elevated (Dalton et al, 2005; Kleinhans et al, 2009; Monk et al, 2010; Tottenham et al, 2013; Weng et al, 2011) and reduced amygdala reactivity (Ashwin et al, 2007; Bookheimer et al, 2008; Corbett et al, 2009; Hadjikhani et al, 2007; Kleinhans et al, 2011; Perlman et al, 2011). Although increased amygdala reactivity has been interpreted as reflecting increased fear or ambiguity in relation to the stimuli presented (eg, Dalton et al, 2005), the finding of reduced amygdala reactivity was previously linked to impaired attention, salience or motivational relevance of facial stimuli in ASD (eg, Bookheimer et al, 2008). In the present study, amygdala reactivity to facial stimuli appeared to be comparable between the AS group and NT controls under placebo. Although the present study does not provide unambiguous evidence for increased salience rather than increased fear during facial emotion processing following oxytocin treatment in the AS group, the notion of increased salience rather than increased fear would predict increased emotion recognition performance, which was indeed the case in the present study. In addition, amygdala reactivity has been shown to be positively associated with eye gaze in ASD (eg, Dalton et al, 2005), and thus might code for the salience of this particular facial region. However, as we had no eye-tracking data in the present study, future experiments are needed to test the proposed mediating role of visual attention for the observed behavioral and neural effects of oxytocin in ASD.
The whole brain analysis revealed significantly increased activity in the temporal pole after oxytocin treatment in individuals with AS. The temporal pole has been associated with inferring the mental state of others (Frith and Singer, 2008), thus potentially representing another candidate region for mediating the positive effects of oxytocin on emotion recognition. In addition, we found increased reactivity to facial stimuli in the superior temporal gyrus, the anterior insula, the inferior frontal lobe, and the Supplementary Motor Area after oxytocin treatment in the AS group. These regions have previously been implicated in face processing (Atkinson and Adolphs, 2011), cognitive and affective empathy (Shamay-Tsoory, 2011), facial mimicry and imitation (Carr et al, 2003), and might thus represent a part of the neural circuitry underlying facial emotion recognition in the present study. It should be noted that most of the exploratory whole brain results have to be interpreted with caution, as most of the effects did not survive a family-wise error correction for multiple testing. In addition, although there were no comparable oxytocin effects in NT controls, future studies employing bigger sample sizes are needed to directly compare the neural effects of oxytocin in typically developing individuals and those with ASD.
To our knowledge, this is the first study showing beneficial effects of intranasal oxytocin on facial emotion recognition in high functioning adults with AS. We provide evidence that the behavioral effect of intranasal oxytocin is pronounced for the processing of eye stimuli. The present results further suggest that intranasal oxytocin enhances the salience of emotional facial stimuli in ASD, which is in line with previous results using neutral faces (Andari et al, 2010; Domes et al, 2013a). The observation that oxytocin modulated the activity in a distributed network of brain regions involved in social cognitive functioning suggests that the effects of oxytocin in social cognition extend beyond a simple modulation of emotional arousal at the level of the amygdala. As oxytocin did not change wakefulness, calmness, and mood over the course of our study, it is unlikely that the effects are due to a general modulation of arousal. Future studies should explicitly test for the specificity of the observed effects for social stimuli as compared with non-social stimuli, and also incorporate clinical control groups with impairments in social cognitive functioning (eg, social anxiety disorder), in order to clarify the specificity of findings with regard to the diagnostic group.
Oxytocin-induced facilitation of emotion recognition and eye contact might be a crucial prerequisite for social approach behavior and intact social cognitive functioning. The present findings are in line with the results of a study with adolescents (Guastella et al, 2009) and suggest that intranasal oxytocin might help to improve a specific deficit in emotion recognition in ASD, ie, shaping motivation and attention for the most informative aspects of social stimuli (Andari et al, 2010).
FUNDING AND DISCLOSURE
This study was supported by the Deutsche Forschungsgemeinschaft, DFG (Do1312/1-1 and Do1312/2-1). The authors declare no conflict of interest.
References
Adolphs R (2010). What does the amygdala contribute to social cognition? Ann N Y Acad Sci 1191: 42–61.
Adolphs R, Sears L, Piven J (2001). Abnormal processing of social information from faces in autism. J Cogn Neurosci 13: 232–240.
Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A (2010). Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA 107: 4389–4394.
Ashwin C, Baron-Cohen S, Wheelwright S, O'Riordan M, Bullmore ET (2007). Differential activation of the amygdala and the 'social brain' during fearful face-processing in Asperger Syndrome. Neuropsychologia 45: 2–14.
Atkinson AP, Adolphs R (2011). The neuropsychology of face perception: beyond simple dissociations and functional selectivity. Philos Trans R Soc Lond B Biol Sci 366: 1726–1738.
Bakermans-Kranenburg MJ, van Ijzendoorn MH (2013). A sociability gene? Meta-analysis of oxytocin receptor genotype effects in humans. Psychiatr Genet (epub ahead of print, doi: 10.1097/YPG.0b013e3283643684).
Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I (2001). The ‘Reading the Mind in the Eyes’ Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry 42: 241–251.
Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E (2008). Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58: 639–650.
Bookheimer SY, Wang AT, Scott A, Sigman M, Dapretto M (2008). Frontal contributions to face processing differences in autism: evidence from fMRI of inverted face processing. J Int Neuropsychol Soc 14: 922–932.
Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL (2003). Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA 100: 5497–5502.
Carter CS (2007). Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res 176: 170–186.
Chawarska K, Volkmar F, Klin A (2010). Limited attentional bias for faces in toddlers with autism spectrum disorders. Arch Gen Psychiatry 67: 178–185.
Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M (2011). Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc Natl Acad Sci USA 108: 19937–19942.
Corbett BA, Carmean V, Ravizza S, Wendelken C, Henry ML, Carter C et al (2009). A functional and structural study of emotion and face processing in children with autism. Psychiatry Res 173: 196–205.
Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH et al (2005). Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci 8: 519–526.
Dawson G, Webb SJ, McPartland J (2005). Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol 27: 403–424.
Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC (2007a). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry 62: 1187–1190.
Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, Herpertz SC (2013a). Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry 74: 164–171.
Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC (2007b). Oxytocin improves ‘mind-reading’ in humans. Biol Psychiatry 61: 731–733.
Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M et al (2010). Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology 35: 83–93.
Domes G, Sibold M, Schulze L, Lischke A, Herpertz SC, Heinrichs M (2012). Intranasal oxytocin increases covert attention to positive social cues. Psychological Medicine 43: 1747–1753.
Domes G, Steiner A, Porges SW, Heinrichs M (2013b). Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology 38: 1198–1202.
Donaldson ZR, Young LJ (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322: 900–904.
Eckman P, Friesen WV (1976) Pictures of Facial Affect. Consulting Psychologists: Palo Alto, CA, USA.
Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM (1993). 3D Statistical Neuroanatomical Models from 305 MRI Volumes. Nuclear Sci Symp Med Imaging Confc Vols 1-3: 1813–1817.
Frith CD, Singer T (2008). The role of social cognition in decision making. Philos Trans R Soc Lond B Biol Sci 363: 3875–3886.
Gamer M, Zurowski B, Buchel C (2010). Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci USA 107: 9400–9405.
Glascher J (2009). Visualization of group inference data in functional neuroimaging. Neuroinformatics 7: 73–82.
Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ et al (2009). Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry 67: 692–694.
Guastella AJ, Mitchell PB, Dadds MR (2008a). Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry 63: 3–5.
Guastella AJ, Mitchell PB, Mathews F (2008b). Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatry 64: 256–258.
Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H (2007). Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp 28: 441–449.
Harms MB, Martin A, Wallace GL (2010). Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychol Rev 20: 290–322.
Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry 54: 1389–1398.
Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L et al (2007). Oxytocin increases retention of social cognition in autism. Biol Psychiatry 61: 498–503.
Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH Jr. (2007). Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett 417: 6–9.
Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S et al (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 25: 11489–11493.
Kleinhans NM, Johnson LC, Richards T, Mahurin R, Greenson J, Dawson G et al (2009). Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. AJ Psychiatry 166: 467–475.
Kleinhans NM, Richards T, Johnson LC, Weaver KE, Greenson J, Dawson G et al (2011). fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage 54: 697–704.
Klin A, Jones W, Schultz R, Volkmar F, Cohen D (2002). Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry 59: 809–816.
Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E (2005). Oxytocin increases trust in humans. Nature 435: 673–676.
Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP (2007). Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry 13: 980–988.
Lischke A, Berger C, Prehn K, Heinrichs M, Herpertz SC, Domes G (2012a). Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology 37: 475–481.
Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M et al (2012b). Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology 37: 1431–1438.
Liu X, Kawamura Y, Shimada T, Otowa T, Koishi S, Sugiyama T et al (2010). Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J Hum Genet 55: 137–141.
Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC et al (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30: 205–223.
Lord C, Rutter M, Le Couteur A (1994). Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24: 659–685.
Marsh AA, Yu HH, Pine DS, Blair RJ (2010). Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berl) 209: 225–232.
Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M (2011). Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 12: 524–539.
Monk CS, Weng SJ, Wiggins JL, Kurapati N, Louro HM, Carrasco M et al (2010). Neural circuitry of emotional face processing in autism spectrum disorders. J Psychiatry Neurosci 35: 105–114.
Pelphrey KA, Morris JP, McCarthy G (2005). Neural basis of eye gaze processing deficits in autism. Brain 128 (Pt 5): 1038–1048.
Perlman SB, Hudac CM, Pegors T, Minshew NJ, Pelphrey KA (2011). Experimental manipulation of face-evoked activity in the fusiform gyrus of individuals with autism. Soc Neurosci 6: 22–30.
Rimmele U, Hediger K, Heinrichs M, Klaver P (2009). Oxytocin makes a face in memory familiar. J Neurosci 29: 38–42.
Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R et al (2003). The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc Lond B Biol Sci 358: 415–427.
Schulze L, Lischke A, Greif J, Herpertz SC, Heinrichs M, Domes G (2011). Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinology 36: 1378–1382.
Shamay-Tsoory SG (2011). The neural bases for empathy. Neuroscientist 17: 18–24.
Snodgrass JG, Corwin J (1988). Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen 117: 34–50.
Steyer R, Schwenkmezger P, Notz P, Eid M (1997) Der Mehrdimensionale Befindlichkeitsfragebogen MDBF [Multidimensional mood questionnaire]. Hogrefe: Göttingen, Germany.
Tottenham N, Hertzig ME, Gillespie-Lynch K, Gilhooly T, Millner AJ, Casey BJ (2013). Elevated amygdala response to faces and gaze aversion in autism spectrum disorder. Soc Cogn Affect Neurosci (epub ahead of print, doi: 10.1093/scan/nst050).
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15: 273–289.
Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M et al (2011). Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333: 104–107.
Wechsler D (1999) Wechsler abbreviated scale of intelligence. The Psychological Corporation: San Antonio, TX, USA.
Weng SJ, Carrasco M, Swartz JR, Wiggins JL, Kurapati N, Liberzon I et al (2011). Neural activation to emotional faces in adolescents with autism spectrum disorders. J Child Psychol Psychiatry 52: 296–305.
Wermter AK, Kamp-Becker I, Hesse P, Schulte-Korne G, Strauch K, Remschmidt H (2010). Evidence for the involvement of genetic variation in the oxytocin receptor gene (OXTR) in the etiology of autistic disorders on high-functioning level. Am J Med Genet B Neuropsychiatr Genet 153B: 629–639.
Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M et al (2005). Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry 58: 74–77.
Zink CF, Meyer-Lindenberg A (2012). Human neuroimaging of oxytocin and vasopressin in social cognition. Horm Behav 61: 400–409.
Acknowledgements
We are grateful to Christoph Berger, Anne Schuckmann, Mandy Otte, Elisabeth Kunz, Karlheinz Hauenstein, Annette Grossmann, and Manuela Sibold for technical support and valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Domes, G., Kumbier, E., Heinrichs, M. et al. Oxytocin Promotes Facial Emotion Recognition and Amygdala Reactivity in Adults with Asperger Syndrome. Neuropsychopharmacol 39, 698–706 (2014). https://doi.org/10.1038/npp.2013.254
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.254
Keywords
This article is cited by
-
The dual neural effects of oxytocin in autistic youth: results from a randomized trial
Scientific Reports (2022)
-
A randomized controlled trial of intranasal oxytocin in Phelan-McDermid syndrome
Molecular Autism (2021)
-
Randomized clinical trial shows no substantial modulation of empathy-related neural activation by intranasal oxytocin in autism
Scientific Reports (2021)
-
A multi-modal MRI analysis of brain structure and function in relation to OXT methylation in maltreated children and adolescents
Translational Psychiatry (2021)
-
Intranasal oxytocin modulates brain responses to voice-identity recognition in typically developing individuals, but not in ASD
Translational Psychiatry (2020)