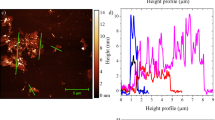
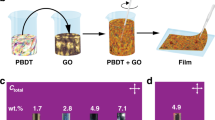
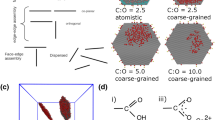
Abstract
Graphene combines unique electronic properties and surprising quantum effects with outstanding thermal and mechanical properties1,2,3,4. Many potential applications, including electronics and nanocomposites, require that graphene be dispersed and processed in a fluid phase5. Here, we show that graphite spontaneously exfoliates into single-layer graphene in chlorosulphonic acid, and dissolves at isotropic concentrations as high as ∼2 mg ml−1, which is an order of magnitude higher than previously reported values. This occurs without the need for covalent functionalization, surfactant stabilization, or sonication, which can compromise the properties of graphene6 or reduce flake size. We also report spontaneous formation of liquid-crystalline phases at high concentrations (∼20–30 mg ml−1). Transparent, conducting films are produced from these dispersions at 1,000Ω □−1 and ∼80% transparency. High-concentration solutions, both isotropic and liquid crystalline, could be particularly useful for making flexible electronics as well as multifunctional fibres.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Segal, M. Selling graphene by the ton. Nature Nanotech. 4, 612–614 (2009).
Novoselov, K. S. et al. Electric field effect transistor in atomically thin carbon film. Science 306, 666–669 (2004).
Lee, C., Wei, X. D., Kysar, J. W. & Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321, 385–388 (2008).
Balandin, A. A. et al. Superior thermal conductivity of single-layer graphene. Nano Lett. 8, 902–907 (2008).
Stankovich, S. et al. Graphene-based composite materials. Nature 442, 282–286 (2006).
Schwamb, T., Burg, B. R., Schirmer, N. C. & Poulikakos, D. An electrical method for the measurement of the thermal and electrical conductivity of reduced graphene oxide nanostructures. Nanotechnology 20, 405704 (2009).
Ruoff, R. Graphene: calling all chemists. Nature Nanotech. 3, 10–11 (2008).
Novoselov, K. S. et al. Two-dimensional atomic crystals. Proc. Natl Acad. Sci. USA 102, 10451–10453 (2005).
Hummers, W. S. & Offeman, R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339 (1958).
Stankovich, S. et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45, 1558–1565 (2007).
Becerril, H. A. et al. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2, 463–470 (2008).
Hernandez, Y. et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nature Nanotech. 3, 563–568 (2008).
Valles, C. et al. Solutions of negatively charged graphene sheets and ribbons. J. Am. Chem. Soc. 130, 15802–15804 (2008).
Lotya, M. et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 131, 3611–3620 (2009).
Davis, V. A. et al. True solutions of single-walled carbon nanotubes for assembly into macroscopic materials. Nature Nanotech. 4, 830–834 (2009).
Cremlyn, R. J. Chlorosulfonic Acid: A Versatile Reagent (The Royal Society of Chemistry, 2002).
Melin, J., Furdin, G., Fuzellier, H., Vasse, R. & Herold, A. Action sur le graphite des solutions de chlorures dans l'acide chlorosulfonique. Mater. Sci. Eng. 31, 61–65 (1977).
Rai, P. K. et al. Isotropic-nematic phase transition of single-walled carbon nanotubes in strong acids. J. Am. Chem. Soc. 128, 591–595 (2006).
Ramesh, S. et al. Dissolution of pristine single walled carbon nanotubes in superacids by direct protonation. J. Phys. Chem. B 108, 8794–8798 (2004).
Sumanasekera, G. U. et al. Electrochemical oxidation of single wall carbon nanotube bundles in sulfuric acid. J. Phys. Chem. B 103, 4292–4297 (1999).
Cancado, L. G. et al. Measuring the degree of stacking order in graphite by Raman spetroscopy. Carbon 46, 272–275 (2008).
Meyer, J. C. et al. On the roughness of single- and bi-layer graphene membranes. Solid State Commun. 143, 101–109 (2007).
Onsager, L. The effects of shape on the interaction of colloidal particles Ann. NY Acad. Sci. 51, 627–659 (1949).
Bates, M. A. & Frenkel, D. Nematic-isotropic transition in polydisperse systems of infinitely thin hard platelets. J. Chem. Phys. 110, 6553–6559 (1999).
van der Kooij, F. M., Kassapidou, K. & Lekkerkerker, H. N. W. Liquid crystal phase transitions in suspensions of polydisperse plate-like particles. Nature 406, 868–871 (2000).
Wensink, H. H. & Vroege, G. J. Isotropic-nematic phase behavior of length-polydisperse hard rods. J. Chem. Phys. 119, 6868–6882 (2003).
Green, M. J., Parra-Vasquez, A. N. G., Behabtu, N. & Pasquali, M. Modeling the phase behavior of polydisperse rigid rods with attractive interactions with applications to single-walled carbon nanotubes in superacids. J. Chem. Phys. 131, 084901 (2009).
van der Beek, D. & Lekkerkerker, H. N. W. Liquid crystal phases of charged colloidal platelets. Langmuir 20, 8582–8586 (2004).
Chandrasekhar, S. Liquid Crystals 2nd edn (Cambridge Univ. Press, 1992).
Campos-Delgado, J. et al. Bulk production of a new form of sp(2) carbon: crystalline graphene nanoribbons. Nano Lett. 8, 2773–2778 (2008).
Jiao, L. Y., Zhang, L., Wang, X. R., Diankov, G. & Dai, H. J. Narrow graphene nanoribbons from carbon nanotubes. Nature 458, 877–880 (2009).
Kosynkin, D. V. et al. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 458, 872–875 (2009).
Talmon, Y. Transmission electron microscopy of complex fluids: the state of the art. Ber. Bunsen Phys. Chem. 100, 364–372 (1996).
Acknowledgements
The authors acknowledge the helpful input of Y. Kauffmann, H. Schmidt, C. Young, M. Majumder, A. Mela, W. Adams and B. Chen. Funding was provided by Air Force Office of Scientific Research (AFOSR) grants FA9550-06-1-0207 and FA9550-09-1-0590, Department of Energy (DOE) (DE-FC-36-05GO15073), Air Force Research Laboratories (AFRL) agreements FA8650-07-2-5061 and 07-S568-0042-01-C1, the Robert A. Welch Foundation (C-1668), US Army Corps of Engineers Environmental Quality and Installation Program under grant W912HZ-08-C-0054, the USA–Israel Binational Science Foundation and the Evans–Attwell Welch Postdoctoral Fellowship. Mitsui & Co. generously donated the MWCNTs used for preparing the nanoribbons. Cryo-TEM was performed at the Electron Microscopy of Soft Matter Laboratory, supported by the Technion Russell Berrie Nanotechnology Institute (RBNI). The HRTEM work was carried out at the Electron Microscopy Center at the Department of Materials Engineering, the Technion.
Author information
Authors and Affiliations
Contributions
J.L. and N.B. conceived, designed and performed the experiments including dispersion and film fabrication. J.L. and A.S. performed AFM. N.B. and D.T. performed and interpreted the Raman measurements. N.B. characterized the liquid crystallinity. N.B. and A.N.G.P.V. designed the HRTEM experiments. A.S. fabricated the electronic devices. N.B., J.L. and A.S. performed electrical measurements. N.B. and A.S. performed SEM. N.B. performed STEM and electron diffraction. N.B. and A.L.H. prepared HRTEM samples, performed HRTEM experiments and interpreted the images. D.K. provided nanoribbons and graphite oxides. Y.T., Y.C., J.S., M.J.G. and E.K. performed HRTEM and cryo-TEM experiments and interpreted the images. N.B., M.J.G., A.L.H., A.S., J.L., Y.T., J.M.T. and M.P. co-wrote the paper. M.P., Y.T., Y.C. and J.M.T. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Behabtu, N., Lomeda, J., Green, M. et al. Spontaneous high-concentration dispersions and liquid crystals of graphene. Nature Nanotech 5, 406–411 (2010). https://doi.org/10.1038/nnano.2010.86
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2010.86
This article is cited by
-
Stable graphene oxide-based lyotropic liquid crystals for interfacial lubrication
Friction (2024)
-
Recent advances of economically synthesised polymers/composites consisting of graphene and silver nanoparticles to achieve sustainable existence
Polymer Bulletin (2024)
-
Graphene oxide for photonics, electronics and optoelectronics
Nature Reviews Chemistry (2023)
-
An overview of contemporary developments and the application of graphene-based materials in anticorrosive coatings
Environmental Science and Pollution Research (2023)
-
Liquid crystalline 2D borophene oxide for inorganic optical devices
Nature Communications (2022)