Abstract
High-resolution optical imaging is critical to understanding brain function. We demonstrate that three-photon microscopy at 1,300-nm excitation enables functional imaging of GCaMP6s-labeled neurons beyond the depth limit of two-photon microscopy. We record spontaneous activity from up to 150 neurons in the hippocampal stratum pyramidale at ∼1-mm depth within an intact mouse brain. Our method creates opportunities for noninvasive recording of neuronal activity with high spatial and temporal resolution deep within scattering brain tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Denk, W., Strickler, J.H. & Webb, W.W. Science 248, 73–76 (1990).
Zipfel, W.R., Williams, R.M. & Webb, W.W. Nat. Biotechnol. 21, 1369–1377 (2003).
Kerr, J.N. & Denk, W. Nat. Rev. Neurosci. 9, 195–205 (2008).
Dombeck, D.A., Khabbaz, A.N., Collman, F., Adelman, T.L. & Tank, D.W. Neuron 56, 43–57 (2007).
Sato, T.R., Gray, N.W., Mainen, Z.F. & Svoboda, K. PLoS Biol. 5, e189 (2007).
Mittmann, W. et al. Nat. Neurosci. 14, 1089–1093 (2011).
Dana, H. et al. eLife 5, e12727 (2016).
Dombeck, D.A., Harvey, C.D., Tian, L., Looger, L.L. & Tank, D.W. Nat. Neurosci. 13, 1433–1440 (2010).
Levene, M.J., Dombeck, D.A., Kasischke, K.A., Molloy, R.P. & Webb, W.W. J. Neurophysiol. 91, 1908–1912 (2004).
Jung, J.C. & Schnitzer, M.J. Opt. Lett. 28, 902–904 (2003).
Ziv, Y. et al. Nat. Neurosci. 16, 264–266 (2013).
Helmchen, F. & Denk, W. Nat. Methods 2, 932–940 (2005).
Theer, P. & Denk, W. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 23, 3139–3149 (2006).
Theer, P., Hasan, M.T. & Denk, W. Opt. Lett. 28, 1022–1024 (2003).
Leray, A., Odin, C., Huguet, E., Amblard, F. & Le Grand, Y. Opt. Commun. 272, 269–278 (2007).
Oheim, M., Beaurepaire, E., Chaigneau, E., Mertz, J. & Charpak, S. J. Neurosci. Methods 111, 29–37 (2001).
Horton, N.G. et al. Nat. Photonics 7, 205–209 (2013).
Andresen, V. et al. Curr. Opin. Biotechnol. 20, 54–62 (2009).
Kobat, D. et al. Opt. Express 17, 13354–13364 (2009).
Kobat, D., Horton, N.G. & Xu, C. J. Biomed. Opt. 16, 106014 (2011).
Chen, T.W. et al. Nature 499, 295–300 (2013).
Chen, I.-H., Chu, S.-W., Sun, C.-K., Cheng, P.-C. & Lin, B.-L. Opt. Quantum Electron. 34, 1251–1266 (2002).
Squier, J., Muller, M., Brakenhoff, G. & Wilson, K.R. Opt. Express 3, 315–324 (1998).
Pologruto, T.A., Sabatini, B.L. & Svoboda, K. Biomed. Eng. Online 2, 13 (2003).
Acknowledgements
We thank members of the Xu and Schaffer/Nishimura research groups for their help and D. Dombeck for discussion. We thank S. Hu for help with histology, the University Institute for Biotechnology, Cornell University for help with Zeiss 780 confocal microscope for imaging fixed brain slices. We thank N. Ji for help with animal preparation methods. The project was supported by DARPA W911NF-14-1-0012 to C.X., NIH/NIBIB R01EB014873 to C.X., and NIH/NINDS U01NS090530 to C.X. It was also supported by the Intelligence Advanced Research Projects Activity (IARPA) via Department of Interior/Interior Business Center (DoI/IBC) contract number D16PC00003 to A.T. and C.X. The US Government is authorized to reproduce and distribute reprints for governmental purposes notwithstanding any copyright annotation thereon. Disclaimer: the views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of IARPA, DoI/IBC, or the US Government.
Author information
Authors and Affiliations
Contributions
C.X. conceived the study. D.G.O., T.W., M.W., D.D.F., N.G.H. J.C.C.-H., and Y.-T.C. performed the experiments. T.W., D.G.O., and J.R. analyzed the data. C.X. and N.N. supervised the project. J.R. and A.S.T. provided transgenic mice for this study. D.G.O., T.W., and C.X. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
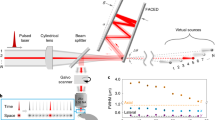
Supplementary Figure 1 Experimental setup
a, Schematic of the excitation sources and the basic optical path of the imaging setup.
b, Measured NOPA output spectrum.
c, Measured interferometric second-order autocorrelation trace of the pulse at the objective focus, with dispersion pre-compensation.
d, Time division multiplexing (TDM) for simultaneous 2PM and 3PM.
M – mirror, HWP – half-wave plate, PBS – polarizing beamsplitter cube, BS – beam stop, DM – dichroic mirror, PMT – photomultiplier tube, S – sampler, NOPA – non-collinear optical amplifier, PD – photo-diode
Supplementary Figure 2 3PM imaging of spontaneous activity in GCaMP6s-labeled neurons in the SP layer of the CA1 region of the mouse hippocampus.
a, 3PM image of neuronal population in the CA1 region of the hippocampus located at 984 μm beneath the dura (left). Neurons are indexed (right) for reference to traces in (b), Supplementary Figure 3 and 4. Average power of 50 mW at 800 kHz repetition rate was used for imaging. The field-of-view (FOV) was 200x200 μm. Scale bar, 20 μm.
b, Spontaneous activity traces recorded from neuron 1-5 in (a) during approximately the first 16 minutes of a 48-minute recording session, at a frame rate of 8.49 Hz. The five neurons represent a range of activity level and brightness. All traces were low-pass filtered with a hamming window with a 0.59 s time constant, and fluorescence intensity was converted to photon counts per neuron per second.
Supplementary Figure 3 Spontaneous activity traces of the hippocampal neurons shown in Supplementary Figure 2
Spontaneous activity traces recorded from all indexed neurons in Supplementary Figure 2 during approximately the first 16 minutes of a 48-minute recording session, at a frame rate of 8.49 Hz. To the left of each trace is the index of the neuron. All traces were low-pass filtered with a hamming window with a 0.59 s time constant, and then normalized to each individual baseline.
Supplementary Figure 4 Continued recording of spontaneous activity traces of the hippocampal neurons after Supplementary Figure 3
Spontaneous activity traces recorded from the same neurons in Supplementary Figure 3 during approximately 16 minutes after 32 minutes of continuous recording (i.e., approximately the last 16 minutes of the 48-minute recording session starting in Supplementary Figure 3). All traces were processed in the same way as in (c).
Supplementary Figure 5 Revisit of the same population of neurons in the SP layer of the CA1 region of the hippocampus after one week.
a, Activity recording site in the SP layer of the hippocampus located at 984 μm beneath the dura (left). The neuronal population was the same as that shown in Figure 2(c) and Supplementary Figure 2, but imaged one week later. Neurons are indexed (right) for reference to their traces in (b) and Supplementary Figure 6. Average power of 58 mW at 800 kHz was used for imaging. The FOV was 150x150 μm. Scale bar 20 μm.
b, Spontaneous activity traces recorded from three indexed neurons in (a) for 10 minutes, at a frame rate of 8.49 Hz. The three neurons represent a range of activity level and brightness. To the left of each trace is the index of the neuron. All traces were low-pass filtered with a hamming window with a 0.59 s time constant, and fluorescent intensity was converted to photon counts per neuron per second.
Supplementary Figure 6 Spontaneous activity traces of the hippocampal neurons shown in Supplementary Figure 5
Spontaneous activity traces recorded from all indexed neurons in Supplementary Figure 5 for 10 minutes, at a frame rate of 8.49 Hz. All traces were low-pass filtered with a hamming window with a 0.59 s time constant, and then normalized to each individual baseline.
Supplementary Figure 7 Histological images of coronal mouse brain sections of the GCaMP6s-labeled site imaged by 3PM in this study
a, Confocal microscopy image of post mortem fixed brain tissue. Scale bar, 1 mm.
b, High resolution ex vivo confocal image from the CA1 region of the hippocampus. Scale bar, 20 μm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1699 kb)
Stack from cortical Layer 5 and 6 (L5/6), through external capsule (EC) to the SP layer of the CA1 region of the hippocampus.
The movie contains 512x512 pixels images combining the fluorescent signal (green) from neurons expressing GCaMP6s with the THG signal (magenta). Depth in μm is displayed on top left corner. Scale bar, 50 μm (AVI 18325 kb)
Spontaneous activity recorded from GCaMP6s-labeled neurons in the SP layer of the CA1 region of the hippocampus.
The movie was recorded with 256x256 pixels image size and 8.49 Hz frame rate at the depth of 984 μm. The movie is accelerated 40 times, and the time is displayed on top left corner. Scale bar, 20 μm. (AVI 24832 kb)
Rights and permissions
About this article
Cite this article
Ouzounov, D., Wang, T., Wang, M. et al. In vivo three-photon imaging of activity of GCaMP6-labeled neurons deep in intact mouse brain. Nat Methods 14, 388–390 (2017). https://doi.org/10.1038/nmeth.4183
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4183
This article is cited by
-
Long-term in vivo three-photon imaging reveals region-specific differences in healthy and regenerative oligodendrogenesis
Nature Neuroscience (2024)
-
Geometric transformation adaptive optics (GTAO) for volumetric deep brain imaging through gradient-index lenses
Nature Communications (2024)
-
The Cousa objective: a long-working distance air objective for multiphoton imaging in vivo
Nature Methods (2024)
-
Miniature three-photon microscopy maximized for scattered fluorescence collection
Nature Methods (2023)
-
Label-free imaging of red blood cells and oxygenation with color third-order sum-frequency generation microscopy
Light: Science & Applications (2023)