Abstract
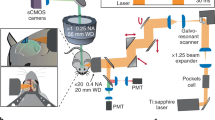
Recent efforts in neuroscience research have been aimed at obtaining detailed anatomical neuronal wiring maps as well as information on how neurons in these networks engage in dynamic activities. Although the entire connectivity map of the nervous system of Caenorhabditis elegans has been known for more than 25 years, this knowledge has not been sufficient to predict all functional connections underlying behavior. To approach this goal, we developed a two-photon technique for brain-wide calcium imaging in C. elegans, using wide-field temporal focusing (WF-TeFo). Pivotal to our results was the use of a nuclear-localized, genetically encoded calcium indicator, NLS-GCaMP5K, that permits unambiguous discrimination of individual neurons within the densely packed head ganglia of C. elegans. We demonstrate near-simultaneous recording of activity of up to 70% of all head neurons. In combination with a lab-on-a-chip device for stimulus delivery, this method provides an enabling platform for establishing functional maps of neuronal networks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Briggman, K.L. & Kristan, W.B. Multifunctional pattern-generating circuits. Annu. Rev. Neurosci. 31, 271–294 (2008).
Bargmann, C.I. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays 34, 458–465 (2012).
Helmstaedter, M. et al. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174 (2013).
Takemura, S.-y. et al. A visual motion detection circuit suggested by Drosophila connectomics. Nature 500, 175–181 (2013).
Niessing, J. & Friedrich, R.W. Olfactory pattern classification by discrete neuronal network states. Nature 465, 47–52 (2010).
Churchland, M.M. et al. Neural population dynamics during reaching. Nature 487, 51–56 (2012).
Ahrens, M.B., Orger, M.B., Robson, D.N., Li, J.M. & Keller, P.J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 10, 413–420 (2013).
Panier, T. et al. Fast functional imaging of multiple brain regions in intact zebrafish larvae using Selective Plane Illumination Microscopy. Front. Neural Circuits 7, 65 (2013).
White, J.G., Southgate, E., Thomson, J.N. & Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B 314, 1–340 (1986).
Grewe, B.F., Langer, D., Kasper, H., Kampa, B.M. & Helmchen, F. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision. Nat. Methods 7, 399–405 (2010).
Katona, G. et al. Fast two-photon in vivo imaging with three-dimensional random-access scanning in large tissue volumes. Nat. Methods 9, 201–208 (2012).
Cheng, A., Gonçalves, J.T., Golshani, P., Arisaka, K. & Portera-Cailliau, C. Simultaneous two-photon calcium imaging at different depths with spatiotemporal multiplexing. Nat. Methods 8, 139–142 (2011).
Turaga, D. & Holy, T.E. Organization of vomeronasal sensory coding revealed by fast volumetric calcium imaging. J. Neurosci. 32, 1612–1621 (2012).
Keller, P.J., Schmidt, A.D., Wittbrodt, J. & Stelzer, E.H.K. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322, 1065–1069 (2008).
Oron, D., Tal, E. & Silberberg, Y. Scanningless depth-resolved microscopy. Opt. Express 13, 1468–1476 (2005).
Zhu, G., van Howe, J., Durst, M., Zipfel, W. & Xu, C. Simultaneous spatial and temporal focusing of femtosecond pulses. Opt. Express 13, 2153–2159 (2005).
Dana, H. & Shoham, S. Numerical evaluation of temporal focusing characteristics in transparent and scattering media. Opt. Express 19, 4937–4948 (2011).
Andrasfalvy, B.K., Zemelman, B.V., Tang, J. & Vaziri, A. Two-photon single-cell optogenetic control of neuronal activity by sculpted light. Proc. Natl. Acad. Sci. USA 107, 11981–11986 (2010).
Vaziri, A., Tang, J., Shroff, H. & Shank, C. Multilayer three-dimensional super-resolution imaging of thick biological samples. Proc. Natl. Acad. Sci. USA 105, 20221–20226 (2008).
Papagiakoumou, E. et al. Scanless two-photon excitation of channelrhodopsin-2. Nat. Methods 7, 848–854 (2010).
Vaziri, A. & Emiliani, V. Reshaping the optical dimension in optogenetics. Curr. Opin. Neurobiol. 22, 128–137 (2012).
Katz, O., Small, E., Bromberg, Y. & Silberberg, Y. Focusing and compression of ultrashort pulses through scattering media. Nat. Photonics 5, 372–377 (2011).
Papagiakoumou, E. et al. Functional patterned multiphoton excitation deep inside scattering tissue. Nat. Photonics 7, 274–278 (2013).
Lyssenko, N.N., Hanna-Rose, W. & Schlegel, R.A. Cognate putative nuclear localization signal effects strong nuclear localization of a GFP reporter and facilitates gene expression studies in Caenorhabditis elegans. Biotechniques 43, 596–600 (2007).
Akerboom, J. et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 32, 13819–13840 (2012).
Bengtson, C.P., Freitag, H.E., Weislogel, J.-M. & Bading, H. Nuclear calcium sensors reveal that repetition of trains of synaptic stimuli boosts nuclear calcium signaling in CA1 pyramidal neurons. Biophys. J. 99, 4066–4077 (2010).
Zimmer, M. et al. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron 61, 865–879 (2009).
Busch, K.E. et al. Tonic signaling from O2 sensors sets neural circuit activity and behavioral state. Nat. Neurosci. 15, 581–591 (2012).
Cáceres, I.C., Valmas, N., Hilliard, M.A. & Lu, H. Laterally orienting C. elegans using geometry at microscale for high-throughput visual screens in neurodegeneration and neuronal development studies. PLoS ONE 7, e35037 (2012).
Chalasani, S.H. et al. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat. Neurosci. 13, 615–621 (2010).
Gray, J.M., Hill, J.J. & Bargmann, C.I. A circuit for navigation in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 102, 3184–3191 (2005).
Hendricks, M., Ha, H., Maffey, N. & Zhang, Y. Compartmentalized calcium dynamics in a C. elegans interneuron encode head movement. Nature 487, 99–103 (2012).
Wu, Y. et al. Inverted selective plane illumination microscopy (iSPIM) enables coupled cell identity lineaging and neurodevelopmental imaging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 108, 17708–17713 (2011).
Palero, J., Santos, S.I.C.O., Artigas, D. & Loza-Alvarez, P. A simple scanless two-photon fluorescence microscope using selective plane illumination. Opt. Express 18, 8491–8498 (2010).
Denk, W. & Detwiler, P.B. Optical recording of light-evoked calcium signals in the functionally intact retina. Proc. Natl. Acad. Sci. USA 96, 7035–7040 (1999).
Lockery, S.R. & Goodman, M.B. The quest for action potentials in C. elegans neurons hits a plateau. Nat. Neurosci. 12, 377–378 (2009).
Liu, Q., Hollopeter, G. & Jorgensen, E.M. Graded synaptic transmission at the Caenorhabditis elegans neuromuscular junction. Proc. Natl. Acad. Sci. USA 106, 10823–10828 (2009).
Chalasani, S.H. et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450, 63–70 (2007).
Piggott, B.J., Liu, J., Feng, Z., Wescott, S.A. & Xu, X.Z.S. The neural circuits and synaptic mechanisms underlying motor initiation in C. elegans. Cell 147, 922–933 (2011).
Chronis, N., Zimmer, M. & Bargmann, C.I. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat. Methods 4, 727–731 (2007).
Kawano, T. et al. An imbalancing act: gap junctions reduce the backward motor circuit activity to bias C. elegans for forward locomotion. Neuron 72, 572–586 (2011).
Qi, Y.B. et al. Hyperactivation of B-type motor neurons results in aberrant synchrony of the Caenorhabditis elegans motor circuit. J. Neurosci. 33, 5319–5325 (2013).
Ferkey, D.M. et al. C. elegans G protein regulator RGS-3 controls sensitivity to sensory stimuli. Neuron 53, 39–52 (2007).
Acknowledgements
We thank J. Akerboom and L. Looger (Howard Hughes Medical Institute, Janelia Farm Research Campus) for valuable information on characteristics of GCaMP variants and for constructs; M. Colombini for manufacturing of mechanical components; E. Sanchez, B. Bathellier, G. Haunert and D. Aschauer for helpful discussions and valuable input; M. Palfreyman, H. Kaplan, S. Kato and C. Bargmann for critically reading the manuscript; R. Latham, M. Sonntag, D. Grzadziela and S. Skora for technical support. R.P. acknowledges the VIPS Program of the Austrian Federal Ministry of Science and Research and the City of Vienna as well as the European Commission (Marie Curie, FP7-PEOPLE-2011-IIF). The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 281869 - elegans Neurocircuits, Vienna Science and Technology Fund (WWTF) project VRG10-11, Human Frontiers Science Program Project RGP0041/2012, Research Platform Quantum Phenomena and Nanoscale Biological Systems (QuNaBioS) and Research Institute of Molecular Pathology (IMP). The IMP is funded by Boehringer Ingelheim.
Author information
Authors and Affiliations
Contributions
T.S. and R.P. designed and performed experiments and analyzed data; R.P. and A.V. designed and built the imaging system; T.S. and M.Z. designed and characterized NLS-GCaMP5K and designed and validated the microfluidic device; K.A. wrote analysis software and analyzed data. M.Z. and A.V. designed experiments and conceived of and led the project. T.S., R.P., M.Z. and A.V. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Table 1 (PDF 1349 kb)
Supplementary Data 1
The data sheets contain the neuron IDs, ΔF/F0 values, raw uncorrected fluorescence traces and elapsed time corresponding to Figures 3-4 and Supplementary Figure 3 (Rows correspond to neuron IDs. Columns correspond to time frames). The correction table assigns to each neuron ID (column A) a reference neuron (column B); see Online Methods. (XLSX 2209 kb)
Supplementary Data 2
The data sheets contain the neuron IDs, ΔF/F0 values, raw uncorrected fluorescence traces and elapsed time corresponding to Figure 5 and Supplementary Figure 9. (Rows correspond to neuron IDs. Columns correspond to time frames). The correction table assigns to each neuron ID (column A) a reference neuron (column B); see Online Methods. (XLSX 1572 kb)
Supplementary Data 3
The data sheets contain the neuron IDs, ΔF/F0 values, raw uncorrected fluorescence traces and elapsed time corresponding to Supplementary Figure 7 (Rows correspond to neuron IDs. Columns correspond to time frames). The correction table assigns to each neuron ID (column A) a reference neuron (column B); see Online Methods. (XLSX 2293 kb)
Supplementary Data 4
The data sheets contain the neuron IDs, ΔF/F0 values, raw uncorrected fluorescence traces and elapsed time corresponding to Supplementary Figure 8 (Rows correspond to neuron IDs. Columns correspond to time frames). The correction table assigns to each neuron ID (column A) a reference neuron (column B); see Online Methods. (XLSX 2676 kb)
Supplementary Data 5
First frame positions of ROIs corresponding to all data sets. (1-4) Images are maximum intensity projections of all z-planes of one recording corresponding to the first acquired volume. Numbered regions indicate positions of all neuron IDs shown in Figures 3, 4, 5, Supplementary Figs 7 and 8. Note, that not all of the neurons are clearly visible at the shown first time point as their fluorescence intensity only increases later during the recording. In some cases regions were slightly moved in order to make all numbers readable. (1) ROIs of recording shown in Figures 3 and 4, Supplementary Figures 3 and 6 (2) ROIs of recording shown in Figure 5, Supplementary Figure 9 (3) ROIs of recording shown in Supplementary Figure 7. (4) ROIs of recording shown in Supplementary Figure 8. (ZIP 5880 kb)
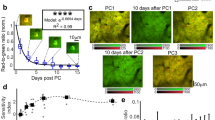
Brain-wide Ca2+−imaging of basal activity in C. elegans.
Maximum intensity projection of 14 z-planes at 2 μm distance of a Punc-31::NLS-GCaMP5K worm. Shown are 200 s recording of basal activity at 21% O2. Frame rate of 70 frames per second equates to 5 volumes per second. See also Figures 3 and 4. (MOV 21488 kb)
Selected sections of brain-wide Ca2+−imaging of basal activity in C. elegans.
Selected transverse- (right) and coronal (bottom) sections plus maximum intensity projection through the left-right axis (center) of 14 z-planes at 2 μm distance of a Punc-31::NLS-GCaMP5K worm. White and yellow lines indicate section planes and projection widths, respectively. Shown are 200 s recording of basal activity at constant 21% O2. Frame rate of 70 frames per second equates to 5 volumes per second. See also Fig. 3 and 4. (MOV 2605 kb)
Brain-wide Ca2+−imaging of neural activity upon repetitive O2 stimuli in C. elegans.
Maximum intensity projection of 16 z-planes at 2 μm distance of a Punc-31::NLS-GCaMP5K worm. Recording time is 232 s. O2 concentrations consecutively shift between 21% and 4% as indicated in the movie. Frame rate of 70 frames per second equates to 4.362 volumes per second. See also Fig. 5. (MOV 2598 kb)
Selected sections of brain-wide Ca2+−imaging of neural activity upon repetitive O2 stimuli in C. elegans.
Selected transverse- (right) and coronal (bottom) sections plus maximum intensity projection through the left-right axis (center) of 16 z-planes at 2 μm distance of a Punc-31::NLS-GCaMP5K worm. White and yellow lines indicate section planes and projection widths, respectively. Recording time is 232 s. O2 concentrations consecutively shift between 21% and 4% as indicated in the movie. The dynamic activity of both BAG and URX neurons (see Fig. 5E, ID 7, 42 and 38, 29) upon O2 shifts can be seen. Frame rate of 70 frames per second equates to 4.362 volumes per second. See also Fig. 5. (MOV 2633 kb)
Rights and permissions
About this article
Cite this article
Schrödel, T., Prevedel, R., Aumayr, K. et al. Brain-wide 3D imaging of neuronal activity in Caenorhabditis elegans with sculpted light. Nat Methods 10, 1013–1020 (2013). https://doi.org/10.1038/nmeth.2637
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2637
This article is cited by
-
Inferring how animals deform improves cell tracking
Nature Methods (2023)
-
Image improvement of temporal focusing multiphoton microscopy via superior spatial modulation excitation and Hilbert–Huang transform decomposition
Scientific Reports (2022)
-
Imaging whole-brain activity to understand behaviour
Nature Reviews Physics (2022)
-
Low-voltage driving high-resistance liquid crystal micro-lens with electrically tunable depth of field for the light field imaging system
Scientific Reports (2022)
-
Large-scale neural recordings call for new insights to link brain and behavior
Nature Neuroscience (2022)