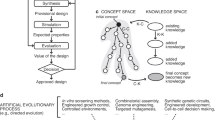
Abstract
Virtual screening is becoming a ground-breaking tool for molecular discovery due to the exponential growth of available computer time and constant improvement of simulation and machine learning techniques. We report an integrated organic functional material design process that incorporates theoretical insight, quantum chemistry, cheminformatics, machine learning, industrial expertise, organic synthesis, molecular characterization, device fabrication and optoelectronic testing. After exploring a search space of 1.6 million molecules and screening over 400,000 of them using time-dependent density functional theory, we identified thousands of promising novel organic light-emitting diode molecules across the visible spectrum. Our team collaboratively selected the best candidates from this set. The experimentally determined external quantum efficiencies for these synthesized candidates were as large as 22%.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Polishchuk, P. G., Madzhidov, T. I. & Varnek, A. Estimation of the size of drug-like chemical space based on GDB-17 data. J. Comput. Aided Mol. Des. 27, 675–679 (2013).
Shoichet, B. K. Virtual screening of chemical libraries. Nature 432, 862–865 (2004).
Curtarolo, S. et al. The high-throughput highway to computational materials design. Nature Mater. 12, 191–201 (2013).
Pyzer-Knapp, E. O., Suh, C., Gómez-Bombarelli, R., Aguilera-Iparraguirre, J. & Aspuru-Guzik, A. What is high-throughput virtual screening? A perspective from organic materials discovery. Annu. Rev. Mater. Res. 45, 195–216 (2015).
Yang, K., Setyawan, W., Wang, S., Buongiorno Nardelli, M. & Curtarolo, S. A search model for topological insulators with high-throughput robustness descriptors. Nature Mater. 11, 614–619 (2012).
Carrete, J., Li, W., Mingo, N., Wang, S. & Curtarolo, S. Finding unprecedentedly low-thermal-conductivity half-Heusler semiconductors via high-throughput materials modeling. Phys. Rev. X 4, 011019 (2014).
Huskinson, B. et al. A metal-free organic–inorganic aqueous flow battery. Nature 505, 195–198 (2014).
Er, S., Suh, C., Marshak, M. P. & Aspuru-Guzik, A. Computational design of molecules for an all-quinone redox flow battery. Chem. Sci. 6, 885–893 (2015).
Hachmann, J. et al. The Harvard Clean Energy Project: large-scale computational screening and design of organic photovoltaics on the world community grid. J. Phys. Chem. Lett. 2, 2241–2251 (2011).
Shin, Y., Liu, J., Quigley, J. J., Luo, H. & Lin, X. Combinatorial design of copolymer donor materials for bulk heterojunction solar cells. ACS Nano 8, 6089–6096 (2014).
Hachmann, J. et al. Lead candidates for high-performance organic photovoltaics from high-throughput quantum chemistry—the Harvard Clean Energy Project. Energy Environ. Sci. 7, 698–704 (2014).
Baldo, M. A. et al. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 395, 151–154 (1998).
Yersin, H. Transition Metal and Rare Earth Compounds 1–26 (Springer, 2004).
Jou, J.-H., Kumar, S., Agrawal, A., Li, T.-H. & Sahoo, S. Approaches for fabricating high efficiency organic light emitting diodes. J. Mater. Chem. C 3, 2974–3002 (2015).
Tao, Y. et al. Thermally activated delayed fluorescence materials towards the breakthrough of organoelectronics. Adv. Mater. 26, 7931–7958 (2014).
Endo, A. et al. Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes. Appl. Phys. Lett. 98, 083302 (2011).
Zhang, Q. et al. Design of efficient thermally activated delayed fluorescence materials for pure blue organic light emitting diodes. J. Am. Chem. Soc. 134, 14706–14709 (2012).
Zhang, Q. et al. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nature Photon. 8, 326–332 (2014).
Yersin, H., Rausch, A. F., Czerwieniec, R., Hofbeck, T. & Fischer, T. The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord. Chem. Rev. 255, 2622–2652 (2011).
Dias, F. B. et al. Triplet harvesting with 100% efficiency by way of thermally activated delayed fluorescence in charge transfer OLED emitters. Adv. Mater. 25, 3707–3714 (2013).
Jankus, V. et al. Highly efficient TADF OLEDs: How the emitter–host interaction controls both the excited state species and electrical properties of the devices to achieve near 100% triplet harvesting and high efficiency. Adv. Funct. Mater. 24, 6178–6186 (2014).
Zhang, Q. et al. Nearly 100% internal quantum efficiency in undoped electroluminescent devices employing pure organic emitters. Adv. Mater. 27, 2096–2100 (2015).
Tanaka, H., Shizu, K., Lee, J. & Adachi, C. Effect of atom substitution in chalcogenodiazole-containing thermally activated delayed fluorescence emitters on radiationless transition. J. Phys. Chem. C 119, 2948–2955 (2015).
Lee, J. et al. Controlled emission colors and singlet–triplet energy gaps of dihydrophenazine-based thermally activated delayed fluorescence emitters. J. Mater. Chem. C 3, 2175–2181 (2015).
Wang, H. et al. Novel thermally activated delayed fluorescence materials–thioxanthone derivatives and their applications for highly efficient OLEDs. Adv. Mater. 26, 5198–5204 (2014).
Lee, D. R., Hwang, S.-H., Jeon, S. K., Lee, C. W. & Lee, J. Y. Benzofurocarbazole and benzothienocarbazole as donors for improved quantum efficiency in blue thermally activated delayed fluorescent devices. Chem. Commun. 51, 8105–8107 (2015).
Shizu, K. et al. Strategy for designing electron donors for thermally activated delayed fluorescence emitters. J. Phys. Chem. C 119, 1291–1297 (2015).
Sagara, Y. et al. Highly efficient thermally activated delayed fluorescence emitters with a small singlet–triplet energy gap and large oscillator strength. Chem. Lett. 44, 360–362 (2015).
Shu, Y. & Levine, B. G. Simulated evolution of fluorophores for light emitting diodes. J. Chem. Phys. 142, 104104 (2015).
Zhang, X. et al. Theoretical investigation of dihydroacridine and diphenylsulphone derivatives as thermally activated delayed fluorescence emitters for organic light-emitting diodes. RSC Adv. 5, 51586–51591 (2015).
RDKit: open source cheminformatics software http://www.rdkit.org (accessed 23 June 2015).
Ertl, P. & Schuffenhauer, A. Estimation of synthetic accessibility score of drug-like molecules based on molecular complexity and fragment contributions. J. Cheminform. 1, 8 (2009).
Baleizão, C. & Berberan-Santos, M. N. Thermally activated delayed fluorescence as a cycling process between excited singlet and triplet states: application to the fullerenes. J. Chem. Phys. 126, 204510 (2007).
Hilborn, R. C. Einstein coefficients, cross sections, f values, dipole moments, and all that. Am. J. Phys. 50, 982–986 (1982).
Dreuw, A. & Head-Gordon, M. Single-reference ab initio methods for the calculation of excited states of large molecules. Chem. Rev. 105, 4009–4037 (2005).
Gritsenko, O. & Baerends, E. J. Asymptotic correction of the exchange–correlation kernel of time-dependent density functional theory for long-range charge-transfer excitations. J. Chem. Phys. 121, 655–660 (2004).
Huang, S. et al. Computational prediction for singlet- and triplet-transition energies of charge-transfer compounds. J. Chem. Theory Comput. 9, 3872–3877 (2013).
Penfold, T. J. On predicting the excited-state properties of thermally activated delayed fluorescence emitters. J. Phys. Chem. C 119, 13535–13544 (2015).
Moral, M., Muccioli, L., Son, W-J., Olivier, Y. & Sancho-García, J. C. Theoretical rationalization of the singlet–triplet gap in OLEDs materials: impact of charge-transfer character. J. Chem. Theory Comput. 11, 168–177 (2015).
Bergeron, C., Krein, M., Moore, G., Breneman, C. M. & Bennett, K. P. Modeling choices for virtual screening hit identification. Mol. Inform. 30, 765–777 (2011).
Eckert, H. & Bajorath, J. Molecular similarity analysis in virtual screening: foundations, limitations and novel approaches. Drug Discov. Today 12, 225–233 (2007).
Dahl, G. E., Jaitly, N. & Salakhutdinov, R. Multi-task neural networks for QSAR predictions. Preprint at http://arXiv.org/abs/1406.1231 (2014).
Rogers, D. & Hahn, M. Extended-connectivity fingerprints. J. Chem. Inf. Model. 50, 742–754 (2010).
Nair, V. & Hinton, G. E. Rectified linear units improve restricted Boltzmann machines. Proc. 27th International Conference on Machine Learning (ICML-10) 807–814 (2010).
Srivastava, N., Hinton, G., Krizhevsky, A., Sutskever, I. & Salakhutdinov, R. Dropout: a simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 15, 1929–1958 (2014).
Snoek, J., Larochelle, H. & Adams, R. P. Practical Bayesian optimization of machine learning algorithms. Adv. Neural Inf. Process. Syst. 25, 2951–2959 (2012).
Maclaurin, D., Duvenaud, D. & Johnson, M. J. HIPS/autograd GitHub https://github.com/HIPS/autograd (accessed 29 October 2015).
Hirata, S. et al. Highly efficient blue electroluminescence based on thermally activated delayed fluorescence. Nature Mater. 14, 330–336 (2015).
Tosco, P., Stiefl, N. & Landrum, G. Bringing the MMFF force field to the RDKit: implementation and validation. J. Cheminformat. 6, 37 (2014).
Shao, Y. et al. Advances in molecular quantum chemistry contained in the Q-Chem 4 program package. Mol. Phys. 113, 184–215 (2015).
Ufimtsev, I. S. & Martinez, T. J. Quantum chemistry on graphical processing units. 3. Analytical energy gradients, geometry optimization, and first principles molecular dynamics. J. Chem. Theory Comput. 5, 2619–2628 (2009).
Acknowledgements
The authors acknowledge Samsung Advanced Institute of Technology for funding and Lumtec Inc. for custom synthesis of candidate materials. The assistance of Samsung for synthesis and characterization of lead compounds is acknowledged. M.A.B.-F. acknowledges support from the DOE Office of Science Graduate Fellowship. M.E. and T.W. were supported by the US Department of Energy, Office of Basic Energy Sciences (Award No. DE-FG02-07ER46474). G.M. thanks the German Academic Exchange Service (DAAD) for a postdoctoral fellowship. The authors acknowledge the use of the Harvard FAS Odyssey Cluster and support from FAS Research Computing.
Author information
Authors and Affiliations
Contributions
A.A.-G., M.B. and R.P.A. conceived the project. T.D.H. designed and wrote the custom computer code for molecular screening, with contributions from R.G.-B. and J.A.-I. R.G.-B. and J.A.-I. designed the molecules, with contributions from A.A.-G., H.M., M.N. and H.S.C. R.G.-B. and J.A.-I. performed calculations and analysed theoretical predictions. M.A.B.-F. carried out the experimental calibration of the theoretical methods. D.M., D.D. and R.P.A. applied machine learning to the computational predictions. H.S.C. and G.M. assessed synthetic feasibility of molecular candidates, with contributions from W.H., S.J., H.M., M.N. and S.K. R.G.-B., J.A.-I., T.D.H., H.S.C., M.A.B.-F., G.M., D.M., D.D., S.H., S.J., H.M., M.N., S.K., R.P.A., M.B. and A.A.-G. selected the molecules for characterization. S.J. synthesized J1-2 and L1. S.J., H.S.C., T.W., D.-G.H. and M.E. collected and analysed spectroscopic data. D.-G.H., M.E. and T.W. manufactured and tested devices for F1, J1, J2 and L1, with contributions from H.K. R.G.-B., J.A.-I. and T.D.H. wrote the first version of the manuscript. All authors contributed to the discussion, writing and editing of the manuscript. A.A.-G. and R.P.A. supervised the computational chemistry study. R.P.A. and A.A.-G. supervised the machine learning approach. M.B. supervised the device fabrication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 7524 kb)
Rights and permissions
About this article
Cite this article
Gómez-Bombarelli, R., Aguilera-Iparraguirre, J., Hirzel, T. et al. Design of efficient molecular organic light-emitting diodes by a high-throughput virtual screening and experimental approach. Nature Mater 15, 1120–1127 (2016). https://doi.org/10.1038/nmat4717
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4717
This article is cited by
-
Autonomous reaction Pareto-front mapping with a self-driving catalysis laboratory
Nature Chemical Engineering (2024)
-
A database of thermally activated delayed fluorescent molecules auto-generated from scientific literature with ChemDataExtractor
Scientific Data (2024)
-
A materials informatics driven fine-tuning of triazine-based electron-transport layer for organic light-emitting devices
Scientific Reports (2024)
-
Interpretable discovery of semiconductors with machine learning
npj Computational Materials (2023)
-
High-throughput property-driven generative design of functional organic molecules
Nature Computational Science (2023)