Abstract
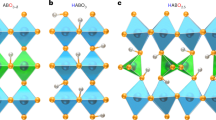
With hydrogen being seen as a key renewable energy vector, the search for materials exhibiting fast hydrogen transport becomes ever more important. Not only do hydrogen storage materials require high mobility of hydrogen in the solid state, but the efficiency of electrochemical devices is also largely determined by fast ionic transport. Although the heavy alkaline-earth hydrides are of limited interest for their hydrogen storage potential, owing to low gravimetric densities, their ionic nature may prove useful in new electrochemical applications, especially as an ionically conducting electrolyte material. Here we show that barium hydride shows fast pure ionic transport of hydride ions (H−) in the high-temperature, high-symmetry phase. Although some conductivity studies have been reported on related materials previously, the nature of the charge carriers has not been determined. BaH2 gives rise to hydride ion conductivity of 0.2 S cm−1 at 630 °C. This is an order of magnitude larger than that of state-of-the-art proton-conducting perovskites or oxide ion conductors at this temperature. These results suggest that the alkaline-earth hydrides form an important new family of materials, with potential use in a number of applications, such as separation membranes, electrochemical reactors and so on.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Schlapbach, L. & Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 414, 353–358 (2001).
Chen, P., Xiong, Z. T., Luo, J. Z., Lin, J. Y. & Tan, K. L. Interaction of hydrogen with metal nitrides and imides. Nature 420, 302–304 (2002).
Zuttel, A. et al. LiBH4 a new hydrogen storage material. J. Power Sources 118, 1–7 (2003).
Blanchard, D., Brinks, H. W., Hauback, B. C. & Norby, P. Desorption of LiAlH4 with Ti- and V-based additives. Mater. Sci. Eng. 108, 54–59 (2004).
Peterson, D. T. & Indig, M. The barium–barium hydride system. J. Am. Chem. Soc. 82, 5645–5646 (1960).
Verbraeken, M. C., Suard, E. & Irvine, J. T. S. Structural and electrical properties of calcium and strontium hydrides. J. Mater. Chem. 19, 2766–2770 (2009).
Bronger, W., Scha, C. C. & Muller, P. Crystal-structure of barium hydride, determined by neutron-diffraction experiments on BaD2 . Z. Anorg. Allg. Chem. 545, 69–74 (1987).
Ting, V. P., Henry, P. F., Kohlmann, H., Wilson, C. C. & Weller, M. T. Structural isotope effects in metal hydrides and deuterides. Phys. Chem. Chem. Phys. 12, 2083–2088 (2010).
Dorfman, S. M. et al. Phase transitions and equations of state of alkaline earth fluorides CaF2, SrF2, and BaF2 to Mbar pressures. Phys. Rev. B 81, 174121 (2010).
Wang, J. S. et al. Structural phase transitions of SrF2 at high pressure. J. Solid State Chem. 186, 231–234 (2012).
Tse, J. S. et al. Structural phase transition in CaH2 at high pressures. Phys. Rev. B 75, 134108 (2007).
Smith, J. S., Desgreniers, S., Klug, D. D. & Tse, J. S. High-density strontium hydride: An experimental and theoretical study. Solid State Commun. 149, 830–834 (2009).
Smith, J. S., Desgreniers, S., Tse, J. S. & Klug, D. D. High-pressure phase transition observed in barium hydride. J. Appl. Phys. 102, 043520 (2007).
Hull, S., Keen, D. A., Sivia, D. S. & Berastegui, P. Crystal structures and ionic conductivities of ternary derivatives of the silver and copper monohalides - I. Superionic phases of stoichiometry MA4I5: RbAg4I5, KAg4I5, and KCu4I5 . J. Solid State Chem. 165, 363–371 (2002).
Gorelov, V. P. & Pal’guev, S. F. Conductivity in CaH2–LiH and CaH2–CaF2 systems. Elektrokhimiya 28, 1294–1296 (1992).
Gibson, I. R. & Irvine, J. T. S. Study of the order–disorder transition in yttria-stabilised zirconia by neutron diffraction. J. Mater. Chem. 6, 895–898 (1996).
Steele, B. C. H. in High Conductivity Solid Ionic Conductors (ed Takahashi, T.) 402–446 (World Scientific Publishing, 1989).
Steele, B. C. H. Appraisal of Ce1−yGdyO2−y/2 electrolytes for IT-SOFC operation at 500 °C. Solid State Ion. 129, 95–110 (2000).
Chen, F. L., Sorensen, O. T., Meng, G. Y. & Peng, D. K. Preparation of Nd-doped BaCeO3 proton-conducting ceramic and its electrical properties in different atmospheres. J. Eur. Ceram. Soc. 18, 1389–1395 (1998).
Omar, S., Wachsman, E. D. & Nino, J. C. Higher conductivity Sm3+ and Nd3+ co-doped ceria-based electrolyte materials. Solid State Ion. 178, 1890–1897 (2008).
Norby, T. Solid-state protonic conductors: Principles, properties, progress and prospects. Solid State Ion. 125, 1–11 (1999).
Hayward, M. A. et al. The hydride anion in an extended transition metal oxide array: LaSrCoO3H0.7 . Science 295, 1882–1884 (2002).
Norby, T., Wideroe, M., Glockner, R. & Larring, Y. Hydrogen in oxides. Dalton Trans. 19, 3012–3018 (2004).
Boukamp, B. A. A linear Kronig–Kramers transform test for immittance data validation. J. Electrochem. Soc. 142, 1885–1894 (1995).
Acknowledgements
We thank EPSRC for support through a Platform Grant, the Royal Society for a Wolfson Merit award and Institut Laue-Langevin for provision of neutron beam time.
Author information
Authors and Affiliations
Contributions
M.C.V. contributed significantly to all aspects, C.C. to synthesis and characterization, E.S. to neutron diffraction and J.T.S.I. to concept and manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 827 kb)
Supplementary Information
Supplementary Information (CIF 25 kb)
Supplementary Information
Supplementary Information (CIF 37 kb)
Supplementary Information
Supplementary Information (CIF 27 kb)
Supplementary Information
Supplementary Information (CIF 25 kb)
Supplementary Information
Supplementary Information (CIF 25 kb)
Supplementary Information
Supplementary Information (CIF 25 kb)
Rights and permissions
About this article
Cite this article
Verbraeken, M., Cheung, C., Suard, E. et al. High H− ionic conductivity in barium hydride. Nature Mater 14, 95–100 (2015). https://doi.org/10.1038/nmat4136
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4136
This article is cited by
-
A simple path to ambient temperature ionic H− superconductors
Science China Chemistry (2024)
-
Deforming lanthanum trihydride for superionic conduction
Nature (2023)
-
Order–disorder and ionic conductivity in calcium nitride-hydride
Nature Communications (2023)
-
High proton conduction in Ba2LuAlO5 with highly oxygen-deficient layers
Communications Materials (2023)
-
Hydride-ion-conducting K2NiF4-type Ba–Li oxyhydride solid electrolyte
Nature Materials (2022)