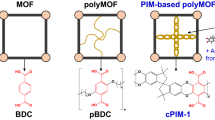
Abstract
Microporous organic polymers (MOPs) are of potential significance for gas storage1,2,3, gas separation4 and low-dielectric applications5. Among many approaches for obtaining such materials, solution-processable MOPs derived from rigid and contorted macromolecular structures are promising because of their excellent mass transport and mass exchange capability. Here we show a class of amorphous MOP, prepared by [2+3] cycloaddition modification of a polymer containing an aromatic nitrile group with an azide compound, showing super-permeable characteristics and outstanding CO2 separation performance, even under polymer plasticization conditions such as CO2/light gas mixtures. This unprecedented result arises from the introduction of tetrazole groups into highly microporous polymeric frameworks, leading to more favourable CO2 sorption with superior affinity in gas mixtures, and selective CO2 transport by presorbed CO2 molecules that limit access by other light gas molecules. This strategy provides a direction in the design of MOP membrane materials for economic CO2 capture processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yaghi, O. M. et al. Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003).
Cooper, A. I. Conjugated microporous polymers. Adv. Mater. 21, 1291–1295 (2009).
McKeown, N. B. et al. Towards polymer-based hydrogen storage materials: Engineering ultramicroporous cavities within polymers of intrinsic microporosity. Angew. Chem. Int. Ed. 45, 1804–1807 (2006).
Park, H. B. et al. Polymers with cavities tuned for fast selective transport of small molecules and ions. Science 318, 254–258 (2007).
Long, T. M. & Swager, T. M. Molecular design of free volume as a route to low-k dielectric materials. J. Am. Chem. Soc. 125, 14113–14119 (2005).
Davis, M. E. Ordered porous materials for emerging applications. Nature 417, 817–821 (2002).
Schüth, F. & Schmidt, W. Microporous and mesoporous materials. Adv. Mater. 14, 629–638 (2002).
Kitagawa, S., Kitaura, R. & Noro, S-I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
Nagai, K., Masuda, T., Nakagawa, T., Freeman, B. D. & Pinnau, I. Poly[1-(trimethylsilyl)-1-propyne] and related polymers: Synthesis, properties and functions. Prog. Polym. Sci. 26, 721–798 (2001).
Park, H. B., Han, S. H., Jung, C. H., Lee, Y. M. & Hill, A. J. Thermally rearranged (TR) polymer membranes for CO2 separation. J. Membr. Sci. 359, 11–24 (2010).
Budd, P. M., McKeown, N. B. & Fritsch, D. Polymers of intrinsic microporosity (PIMs): High free volume polymers for membrane applications. Macromol. Sym. 245, 403–405 (2006).
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
El-Kaderi, H. M. et al. Designed synthesis of 3D covalent organic frameworks. Science 316, 268–272 (2007).
Budd, P. M. et al. Gas separation membranes from polymers of intrinsic microporosity. J. Membr. Sci. 251, 263–269 (2005).
Du, N. Y., Robertson, G. P. & Guiver, M. D. Polymers of intrinsic microporosity derived from novel disulfone-based monomers. Macromolecules 42, 6023–6030 (2009).
Du, N. Y., Robertson, G. P., Song, J. S. & Guiver, M. D. High-performance carboxylated polymers of intrinsic microporosity (PIMs) with tunable gas transport properties. Macromolecules 42, 6038–6043 (2009).
Du, N. Y. et al. Polymers of intrinsic microporosity containing trifluoromethyl and phenylsulfone groups as materials for membrane gas separation. Macromolecules 41, 9656–9662 (2008).
Robeson, L. M. The upper bound revisited. J. Membr. Sci. 320, 390–400 (2008).
Lin, H. & Freeman, B. D. Materials selection guideline for membranes that remove CO2 from gas mixtures. J. Mol. Struct. 739, 57–74 (2005).
Lin, H. & Freeman, B. D. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 239, 105–117 (2004).
Vogiatzis, K., Mavrandonakis, A., Klopper, W. & Froudakis, G. E. Ab initio study of the interactions between CO2 and N-containing organic heterocycles. ChemPhysChem 10, 374–383 (2009).
Gothelf, K. V. & Jørgensen, K. A. Asymmetric 1,3-dipolar cycloaddition reactions. Chem. Rev. 98, 863–910 (1998).
Huisgen, R., Szeimies, G. & Möbius, L. 1,3-Dipolar cycloadditions. XXXII. Kinetics of the addition of organic azides to carbon-carbon multiple bonds. Chem. Ber. 100, 2494–2507 (1967).
Binder, W. H. & Sachsenhofer, R. Click chemistry in polymer and materials science. Macromol. Rapid Commun. 28, 15–54 (2007).
Guiver, M. D., Robertson, G. P., Yoshikawa, M. & Tam, C. M. Functionalized polysulfones: Methods for chemical modification and membrane applications. ACS Symp. Ser. 744, 137–161 (2000) Chapter 10.
Guiver, M. D. & Robertson, G. P. US Patent 5,475,065 (1995).
Tsarevsky, N. V., Bernaerts, K. V., Dufour, B., Du Prez, F. E. & Matyjaszewski, K. Well-defined (co)polymers with 5-vinyltetrazole units via combination of atom transfer radical (co)polymerization of acrylonitrile and click chemistry-type postpolymerization modification. Macromolecules 37, 9308–9313 (2004).
Pinnau, I., Casillas, C. G., Morisato, A. & Freeman, B. D. Hydrocarbon/hydrogen mixed gas permeation in poly(1-trimethylsilyl-1-propyne) (PTMSP), poly(1-phenyl-1-propyne) (PPP), and PTMSP/PPP blends. J. Polym. Sci. Polym. Phys. 34, 2613–2621 (1996).
Thomas, S., Pinnau, I., Du, N. & Guiver, M. D. Hydrocarbon/hydrogen mixed-gas permeation properties of PIM-1, an amorphous microprous spirobisindane polymer. J. Membr. Sci. 338, 1–4 (2009).
Merkel, T., Lin, H., Wei, X. & Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 359, 126–139 (2010).
Figueroa, J. D., Fout, T., Plasynski, S., McIlvried, H. & Srivastava, R. D. Advances in CO2 capture technology—the US Department of Energy’s Carbon Sequestration Program. Int. J. Greenhouse Gas Control 2, 9–20 (2008).
Acknowledgements
NRCC No. 52847. The authors acknowledge partial support from the Climate Change Technology and Innovation Initiative, Greenhouse Gas project (CCTII, GHG), Natural Resources Canada (NRCan) and from Vaperma. H.B.P. and M.D.G. acknowledge support by the WCU (World Class University) programme through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (No. R31-2008-000-10092-0). The authors are very grateful to F. Toll of the National Research Council for the BET absorption measurements.
Author information
Authors and Affiliations
Contributions
N.D. experimental design, synthesis and gas permeation experiments, data analysis, manuscript writing; H.B.P. computer modeling, gas permeation and gas adsorption experiments, data analysis, manuscript writing; G.P.R. NMR and TGA-MS experiments, data analysis; M.M.D-C. gas permeation experiments, data analysis; T.V. industrial application input; L.S. assisted in the synthetic experiments; M.D.G. project idea, direction and supervision, experimental design, data analysis, manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 794 kb)
Rights and permissions
About this article
Cite this article
Du, N., Park, H., Robertson, G. et al. Polymer nanosieve membranes for CO2-capture applications. Nature Mater 10, 372–375 (2011). https://doi.org/10.1038/nmat2989
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2989
This article is cited by
-
Sub-micro porous thin polymer membranes for discriminating H2 and CO2
Nature Communications (2024)
-
Non-solvent post-modifications with volatile reagents for remarkably porous ketone functionalized polymers of intrinsic microporosity
Nature Communications (2023)
-
Remarkably Improved Gas Separation Performance of Polyimides by Forming “Bent and Battered” Main Chain Using Paracyclophane as Building Block
Chinese Journal of Polymer Science (2023)
-
Low Pt loading for high-performance fuel cell electrodes enabled by hydrogen-bonding microporous polymer binders
Nature Communications (2022)
-
Ultra-selective molecular-sieving gas separation membranes enabled by multi-covalent-crosslinking of microporous polymer blends
Nature Communications (2021)