Abstract
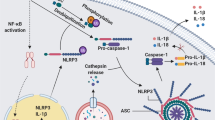
Low-grade, chronic inflammation has been associated with many diseases of aging, but the mechanisms responsible for producing this inflammation remain unclear. Inflammasomes can drive chronic inflammation in the context of an infectious disease or cellular stress, and they trigger the maturation of interleukin-1β (IL-1β). Here we find that the expression of specific inflammasome gene modules stratifies older individuals into two extremes: those with constitutive expression of IL-1β, nucleotide metabolism dysfunction, elevated oxidative stress, high rates of hypertension and arterial stiffness; and those without constitutive expression of IL-1β, who lack these characteristics. Adenine and N4-acetylcytidine, nucleotide-derived metabolites that are detectable in the blood of the former group, prime and activate the NLRC4 inflammasome, induce the production of IL-1β, activate platelets and neutrophils and elevate blood pressure in mice. In individuals over 85 years of age, the elevated expression of inflammasome gene modules was associated with all-cause mortality. Thus, targeting inflammasome components may ameliorate chronic inflammation and various other age-associated conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Howcroft, T.K. et al. The role of inflammation in age-related disease. Aging 5, 84–93 (2013).
Okin, D. & Medzhitov, R. Evolution of inflammatory diseases. Curr. Biol. 22, R733–R740 (2012).
Scrivo, R., Vasile, M., Bartosiewicz, I. & Valesini, G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun. Rev. 10, 369–374 (2011).
Kotas, M.E. & Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015).
Proctor, M.J. et al. Systemic inflammation predicts all-cause mortality: a Glasgow inflammation outcome study. PLoS One 10, e0116206 (2015).
Arai, Y. et al. Inflammation, but not telomere length, predicts successful ageing at extreme old age: a longitudinal study of semi-supercentenarians. EBioMedicine 2, 1549–1558 (2015).
Shen-Orr, S.S. et al. Defective signaling in the JAK-STAT pathway tracks with chronic inflammation and cardiovascular risk in aging humans. Cell Syst. 3, 374–384 (2016).
Furman, D. et al. Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol. Syst. Biol. 9, 659 (2013).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Zitvogel, L., Kepp, O., Galluzzi, L. & Kroemer, G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat. Immunol. 13, 343–351 (2012).
Youm, Y.H. et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 18, 519–532 (2013).
Sardi, F. et al. Alzheimer's disease, autoimmunity and inflammation. The good, the bad and the ugly. Autoimmun. Rev. 11, 149–153 (2011).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426 (2002).
Song, F., Ma, Y., Bai, X.Y. & Chen, X. The expression changes of inflammasomes in the aging rat kidneys. J. Gerontol. A Biol. Sci. Med. Sci. 71, 747–756 (2016).
Furman, D. et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA 111, 869–874 (2014).
Wang, C. et al. Effects of aging, cytomegalovirus infection, and EBV infection on human B cell repertoires. J. Immunol. 192, 603–611 (2014).
Furman, D. et al. Cytomegalovirus infection enhances the immune response to influenza. Sci. Transl. Med. 7, 281ra43 (2015).
Segal, E. et al. Module networks: identifying regulatory modules and their condition-specific regulators from gene expression data. Nat. Genet. 34, 166–176 (2003).
Benjamini, Y.H.Y. Controlling the false Discovery rate: a practical and powerful approach to multiple testing. J Royal Statistical Society. 57, 289–300 (1995).
Huang, W., Sherman, B.T. & Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Yaari, G., Bolen, C.R., Thakar, J. & Kleinstein, S.H. Quantitative set analysis for gene expression: a method to quantify gene set differential expression including gene-gene correlations. Nucleic Acids Res. 41, e170 (2013).
Nwankwo, T., Yoon, S.S., Burt, V. & Gu, Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief 133, 1–8 (2013).
Tusher, V.G., Tibshirani, R. & Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98, 5116–5121 (2001).
Xia, J. & Wishart, D.S. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 26, 2342–2344 (2010).
Ouyang, X. et al. Adenosine is required for sustained inflammasome activation via the AA receptor and the HIF-1α pathway. Nat. Commun. 4, 2909 (2013).
Niwa, T., Takeda, N. & Yoshizumi, H. RNA metabolism in uremic patients: accumulation of modified ribonucleosides in uremic serum. Technical note. Kidney Int. 53, 1801–1806 (1998).
Gkaliagkousi, E., Passacquale, G., Douma, S., Zamboulis, C. & Ferro, A. Platelet activation in essential hypertension: implications for antiplatelet treatment. Am. J. Hypertens. 23, 229–236 (2010).
Hottz, E.D. et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 122, 3405–3414 (2013).
Minuz, P. et al. Determinants of platelet activation in human essential hypertension. Hypertension 43, 64–70 (2004).
Preston, R.A. et al. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 41, 211–217 (2003).
Kirabo, A. et al. DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Invest. 124, 4642–4656 (2014).
Nelson, D.E. et al. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science 306, 704–708 (2004).
Dai, H., Leeder, J.S. & Cui, Y. A modified generalized Fisher method for combining probabilities from dependent tests. Front. Genet. 5, 32 (2014).
Lelo, A., Miners, J.O., Robson, R. & Birkett, D.J. Assessment of caffeine exposure: caffeine content of beverages, caffeine intake, and plasma concentrations of methylxanthines. Clin. Pharmacol. Ther. 39, 54–59 (1986).
Staehli, F. et al. NLRC5 deficiency selectively impairs MHC class I- dependent lymphocyte killing by cytotoxic T cells. J. Immunol. 188, 3820–3828 (2012).
Dörffel, Y. et al. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension 34, 113–117 (1999).
Fearon, W.F. & Fearon, D.T. Inflammation and cardiovascular disease: role of the interleukin-1 receptor antagonist. Circulation 117, 2577–2579 (2008).
Lamkanfi, M. & Dixit, V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 28, 137–161 (2012).
Zhang, C. et al. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension 60, 154–162 (2012).
Omi, T. et al. An intronic variable number of tandem repeat polymorphisms of the cold-induced autoinflammatory syndrome 1 (CIAS1) gene modifies gene expression and is associated with essential hypertension. Eur. J. Hum. Genet. 14, 1295–1305 (2006).
Johansson, Å. et al. NLRC4 inflammasome is an important regulator of Interleukin-18 levels in patients with acute coronary syndromes: genome-wide association study in the PLATelet inhibition and patient outcomes trial (PLATO). Circ Cardiovasc Genet 8, 498–506 (2015).
Zeller, T. et al. Molecular characterization of the NLRC4 expression in relation to Interleukin-18 levels. Circ Cardiovasc Genet 8, 717–726 (2015).
Crippa, A., Discacciati, A., Larsson, S.C., Wolk, A. & Orsini, N. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am. J. Epidemiol. 180, 763–775 (2014).
Zhou, R., Yazdi, A.S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Thompson, D.M., Lu, C., Green, P.J. & Parker, R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA 14, 2095–2103 (2008).
Rauch, I. et al. NAIP proteins are required for cytosolic detection of specific bacterial ligands in vivo. J. Exp. Med. 213, 657–665 (2016).
Heng, T.S. & Painter, M.W. The immunological genome project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statistical Society, Series B B-57, 289–300 (1995).
Reference Values for Arterial Stiffness' Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. Eur. Heart J. 31, 2338–2350 (2010).
Shin, S.Y. et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 46, 543–550 (2014).
Kanehisa, M. et al. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 42, D199–D205 (2014).
Ernst, J. & Bar-Joseph, Z. STEM: a tool for the analysis of short time series gene expression data. BMC Bioinformatics 7, 191 (2006).
Faustin, B. et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell 25, 713–724 (2007).
Acknowledgements
We thank the Stanford–Ellison longitudinal cohort volunteers for their participation; Project/Regulatory/Data Manager S. Mackey; Research Nurses S. Swope, C. Walsh, S. French, B. Sullivan, S. Cathey, T. Trela and N. Mastman; Clinical Research Associates A. Goel, T. Quan, K. Span, R. Fleischmann, B. Tse, I. Chang and S. Batra. We also are grateful to The Ellison Medical Foundation for initial support and to the NIH (U19 AI090019) and the Howard Hughes Medical Institute for the remainder (M.M.D.). We also thank H. Maecker and Y. Rosenberg-Hasson (Human Immune Monitoring Core) at Stanford, and R.E. Vance and I. Rauch at the University of California, Berkeley, for kindly providing us with material from NLRC4 and caspase-1 knockout mice. B.F., J.D-M. and J.F.M. were funded by Fondation pour la Recherche Médicale (DEQ20110421287), INCa-Cancéropôle GSO, Ligue contre le Cancer de la Dordogne, and the Conseil Régional d'Aquitaine. We thank the Metabolon Inc. for the metabolite analysis.
Author information
Authors and Affiliations
Contributions
Gene expression data were generated at the Human Immune Monitoring Core (Stanford University). D.F. and B.F. conducted stimulation, inhibition and qPCR assays; D.F., C.R.B. and V.J. analyzed data; D.F., B.F., S.D., L.L., I.D. and P.B, designed or conducted in vitro studies of monocyte and platelet activation; J.C. and C.J.K. helped with the design and conducted the hypertension studies in mice; D.F., B.G., E.A.G., G.P.N., G.K.F. and M.H.S. designed or conducted the mass cytometry studies F.H. supervised cardiovascular phenotyping data generation; J.-F.M. and J.D.-M. helped with clinical insights and discussion; C.L.D. coordinated, organized and conducted the clinical studies and contributed to study design; G.P.N. and M.M.D. provided support, contributed with the planning of the immunological studies and contributed to study design; D.F., M.M.D. and B.F. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Figures 1–9 and Supplementary Tables 1–5 (PDF 6724 kb)
Rights and permissions
About this article
Cite this article
Furman, D., Chang, J., Lartigue, L. et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med 23, 174–184 (2017). https://doi.org/10.1038/nm.4267
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4267
This article is cited by
-
Structural basis of the human NAIP/NLRC4 inflammasome assembly and pathogen sensing
Nature Structural & Molecular Biology (2024)
-
Dissecting the oncogenic properties of essential RNA-modifying enzymes: a focus on NAT10
Oncogene (2024)
-
Immune and inflammatory mechanisms in hypertension
Nature Reviews Cardiology (2024)
-
N4-acetylcytidine modifies primary microRNAs for processing in cancer cells
Cellular and Molecular Life Sciences (2024)
-
Exercise suppresses neuroinflammation for alleviating Alzheimer’s disease
Journal of Neuroinflammation (2023)