Abstract
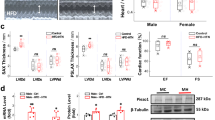
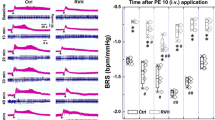
In view of the high proportion of individuals with resistance to antihypertensive medication and/or poor compliance or tolerance of this medication, new drugs to treat hypertension are urgently needed. Here we show that peripheral chemoreceptors generate aberrant signaling that contributes to high blood pressure in hypertension. We discovered that purinergic receptor P2X3 (P2rx3, also known as P2x3) mRNA expression is upregulated substantially in chemoreceptive petrosal sensory neurons in rats with hypertension. These neurons generate both tonic drive and hyperreflexia in hypertensive (but not normotensive) rats, and both phenomena are normalized by the blockade of P2X3 receptors. Antagonism of P2X3 receptors also reduces arterial pressure and basal sympathetic activity and normalizes carotid body hyperreflexia in conscious rats with hypertension; no effect was observed in rats without hypertension. We verified P2X3 receptor expression in human carotid bodies and observed hyperactivity of carotid bodies in individuals with hypertension. These data support the identification of the P2X3 receptor as a potential new target for the control of human hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Go, A.S. et al. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129, 399–410 (2014).
Lloyd, A., Schmieder, C. & Marchant, N. Financial and health costs of uncontrolled blood pressure in the United Kingdom. Pharmacoeconomics 21 (Suppl. 1), 33–41 (2003).
Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360, 1903–1913 (2002).
Kearney, P.M. et al. Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223 (2005).
Carey, R.M. Resistant hypertension. Hypertension 61, 746–750 (2013).
Plump, A. Accelerating the pulse of cardiovascular R&D. Nat. Rev. Drug Discov. 9, 823–824 (2010).
Brown, M.J. Aliskiren. Circulation 118, 773–784 (2008).
Krum, H. et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373, 1275–1281 (2009).
Heusser, K. et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension 55, 619–626 (2010).
Patel, N.K. et al. Deep brain stimulation relieves refractory hypertension. Neurology 76, 405–407 (2011).
Burchell, A.E., Lobo, M.D., Sulke, N., Sobotka, P.A. & Paton, J.F. Arteriovenous anastomosis: is this the way to control hypertension? Hypertension 64, 6–12 (2014).
Lobo, M.D. et al. Central arteriovenous anastomosis for the treatment of patients with uncontrolled hypertension (the ROX CONTROL HTN study): a randomised controlled trial. Lancet 385, 1634–1641 (2015).
Narkiewicz, K. et al. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation 97, 943–945 (1998).
Tan, Z.Y. et al. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ. Res. 106, 536–545 (2010).
Przybylski, J. Do arterial chemoreceptors play a role in the pathogenesis of hypertension? Med. Hypotheses 7, 127–131 (1981).
Somers, V.K., Mark, A.L. & Abboud, F.M. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension 11, 608–612 (1988).
Trzebski, A., Tafil, M., Zoltowski, M. & Przybylski, J. Increased sensitivity of the arterial chemoreceptor drive in young men with mild hypertension. Cardiovasc. Res. 16, 163–172 (1982).
Siński, M. et al. Tonic activity of carotid body chemoreceptors contributes to the increased sympathetic drive in essential hypertension. Hypertens. Res. 35, 487–491 (2012).
Abdala, A.P. et al. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J. Physiol. (Lond.) 590, 4269–4277 (2012).
McBryde, F.D. et al. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat. Commun. 4, 2395 (2013).
Paton, J.F. et al. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61, 5–13 (2013).
Nakayama, K. Surgical removal of the carotid body for bronchial asthma. Dis. Chest 40, 595–604 (1961).
Yuan, G. et al. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci. Signal. 8, ra37 (2015).
Prabhakar, N.R. & Peers, C. Gasotransmitter regulation of ion channels: a key step in O2 sensing by the carotid body. Physiology (Bethesda) 29, 49–57 (2014).
Buckler, K.J. TASK channels in arterial chemoreceptors and their role in oxygen and acid sensing. Pflugers Arch. 467, 1013–1025 (2015).
Evans, A.M., Peers, C., Wyatt, C.N., Kumar, P. & Hardie, D.G. Ion channel regulation by the LKB1-AMPK signalling pathway: the key to carotid body activation by hypoxia and metabolic homeostasis at the whole body level. Adv. Exp. Med. Biol. 758, 81–90 (2012).
Schultz, H.D., Marcus, N.J. & Del Rio, R. Mechanisms of carotid body chemoreflex dysfunction during heart failure. Exp. Physiol. 100, 124–129 (2015).
Ford, A.P. et al. P2X3 receptors and sensitization of autonomic reflexes. Auton. Neurosci. 191, 16–24 (2015).
Abdulqawi, R. et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 385, 1198–1205 (2015).
Zhang, M., Zhong, H., Vollmer, C. & Nurse, C.A. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J. Physiol. (Lond.) 525, 143–158 (2000).
Varas, R., Alcayaga, J. & Iturriaga, R. ACh and ATP mediate excitatory transmission in cat carotid identified chemoreceptor units in vitro. Brain Res. 988, 154–163 (2003).
Zapata, P. Is ATP a suitable co-transmitter in carotid body arterial chemoreceptors? Respir. Physiol. Neurobiol. 157, 106–115 (2007).
Burnstock, G. Purines and sensory nerves. Handb. Exp. Pharmacol. 194, 333–392 (2009).
Icekson, G., Dominguez, C.V., Dedios, V.P., Arroyo, J. & Alcayaga, J. Petrosal ganglion responses to acetylcholine and ATP are enhanced by chronic normobaric hypoxia in the rabbit. Respir. Physiol. Neurobiol. 189, 624–631 (2013).
Kåhlin, J. et al. The human carotid body releases acetylcholine, ATP and cytokines during hypoxia. Exp. Physiol. 99, 1089–1098 (2014).
Prasad, M. et al. Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: role in chemosensory signalling. J. Physiol. (Lond.) 537, 667–677 (2001).
Rong, W. et al. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J. Neurosci. 23, 11315–11321 (2003).
Welsh, M.J., Heistad, D.D. & Abboud, F.M. Depression of ventilation by dopamine in man. Evidence for an effect on the chemoreceptor reflex. J. Clin. Invest. 61, 708–713 (1978).
Cardenas, H. & Zapata, P. Dopamine-induced ventilatory depression in the rat, mediated by carotid nerve afferents. Neurosci. Lett. 24, 29–33 (1981).
Gever, J.R. et al. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br. J. Pharmacol. 160, 1387–1398 (2010).
Clarke, J.A., Daly, M.D. & Ead, H.W. Vascular analysis of the carotid body in the spontaneously hypertensive rat. Adv. Exp. Med. Biol. 337, 3–8 (1993).
Nurse, C.A. Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors. J. Physiol. (Lond.) 592, 3419–3426 (2014).
Fan, J. et al. Interleukin-6 increases intracellular Ca2+ concentration and induces catecholamine secretion in rat carotid body glomus cells. J. Neurosci. Res. 87, 2757–2762 (2009).
Ford, A.P. & Undem, B.J. The therapeutic promise of ATP antagonism at P2X3 receptors in respiratory and urological disorders. Front. Cell. Neurosci. 7, 267 (2013).
Daly, D.M. et al. Age-related changes in afferent pathways and urothelial function in the male mouse bladder. J. Physiol. (Lond.) 592, 537–549 (2014).
Ford, A.P. & Cockayne, D.A. ATP and P2X purinoceptors in urinary tract disorders. Handb. Exp. Pharmacol. 202, 485–526 (2011).
Adriaensen, D., Brouns, I. & Timmermans, J.P. Sensory input to the central nervous system from the lungs and airways: A prominent role for purinergic signalling via P2X2/3 receptors. Auton. Neurosci. 191, 39–47 (2015).
Burnstock, G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal. 10, 3–50 (2014).
Deiteren, A. et al. P2X3 receptors mediate visceral hypersensitivity during acute chemically-induced colitis and in the post-inflammatory phase via different mechanisms of sensitization. PLoS One 10, e0123810 (2015).
Li, J., Xing, J. & Lu, J. Nerve growth factor, muscle afferent receptors and autonomic responsiveness with femoral artery occlusion. J. Mod. Physiol. Res 1, 1–18 (2014).
Hansen, R.R. et al. Chronic administration of the selective P2X3, P2X2/3 receptor antagonist, A-317491, transiently attenuates cancer-induced bone pain in mice. Eur. J. Pharmacol. 688, 27–34 (2012).
Liu, M. et al. Coexpression of P2X(3) and P2X(2) receptor subunits in varying amounts generates heterogeneous populations of P2X receptors that evoke a spectrum of agonist responses comparable to that seen in sensory neurons. J. Pharmacol. Exp. Ther. 296, 1043–1050 (2001).
Gerevich, Z. et al. Dual effect of acid pH on purinergic P2X3 receptors depends on the histidine 206 residue. J. Biol. Chem. 282, 33949–33957 (2007).
Reichling, D.B. & Levine, J.D. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 32, 611–618 (2009).
Schiavuzzo, J.G. et al. Muscle hyperalgesia induced by peripheral P2X3 receptors is modulated by inflammatory mediators. Neuroscience 285, 24–33 (2015).
Zoccal, D.B. et al. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J. Physiol. (Lond.) 586, 3253–3265 (2008).
Moraes, D.J., Machado, B.H. & Paton, J.F. Specific respiratory neuron types have increased excitability that drive presympathetic neurones in neurogenic hypertension. Hypertension 63, 1309–1318 (2014).
Somers, V.K., Mark, A.L. & Abboud, F.M. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J. Clin. Invest. 87, 1953–1957 (1991).
Comroe, J.H. Jr. The functions of the lung. Harvey Lect. 48, 110–143 (1952-1953).
Paton, J.F.R. A working heart-brainstem preparation of the mouse. J. Neurosci. Methods 65, 63–68 (1996).
Moraes, D.J. et al. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J. Neurosci. 33, 19223–19237 (2013).
Waynforth, H.B. & Flecknell, P. Experimental and surgical techniques in the rat. 2nd edn.382 (Academic Press, London, 1992).
Stickland, M.K. et al. Carotid chemoreceptor modulation of blood flow during exercise in healthy humans. J. Physiol. (Lond.) 589, 6219–6230 (2011).
Acknowledgements
We wish to thank J.-C. Isner (School of Biological Sciences, University of Bristol) for his expertise on software used for analyzing aspects of some of the in vivo cardiovascular data. Technical support from P. Chappell (mechanical workshop) and D. Carr (electronic workshop) is appreciated. The research support of the British Heart Foundation RG/12/6/29670 (J.F.R.P.), Afferent Pharmaceuticals (A.P.F. & J.F.R.P.) and the James Tudor Foundation (J.F.R.P.) are acknowledged. This research was supported by the National Institute for Health Research (NIHR) Biomedical Research Unit in Cardiovascular Disease at the University Hospitals Bristol National Health Service Foundation Trust and the University of Bristol (A.K.N. & J.F.R.P.). Studies in situ were supported by grants from 'Fundação de Amparo à Pesquisa do Estado de São Paulo' FAPESP Thematic Project 2013/06077-5 (B.H.M.) and research grant 2013/10484-5 (D.J.A.M.). The University of Bristol's Wolfson Bioimaging Facility BBSRC Alert 13 capital grant BB/L014181/1 is acknowledged. This project also received funding from the Marie Curie International Research Staff Exchange Scheme within the 7th European Community Framework Program under grant agreement no. 612280. This article presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Contributions
W.P. conducted all in vivo radiotelemetry blood pressure studies and the rat and human immunocytochemistry and western blotting; this also included data analysis and figure and manuscript preparation. D.J.A.M. performed all in situ rat nerve and petrosal neuron whole-cell recording studies; this also included data analysis and figure preparation. M.P.d.S. performed the single-neuron PCR study. L.E.K.R. with A.K.N. carried out the diagnosis and recruitment of humans with hypertension, and L.E.K.R. with E.C.H. performed and analyzed data from the dopamine-infusion study. B.H.M. supported all in situ studies and assisted in experimental design, data analysis and manuscript preparation. F.D.M. conducted the in vivo radiotelemetry study for recording renal sympathetic nerve activity; this also included data analysis and figure preparation. A.P.A. performed some of the first immunohistochemistry on human carotid bodies. A.P.F. provided the P2X3-receptor antagonists, carried out the pharmacokinetic analysis and assisted in drug trial design and manuscript preparation and revision. J.F.R.P. orchestrated the design of the project, provided supervision with data acquisition and analysis, wrote the manuscript and revised it, and raised the funding.
Corresponding author
Ethics declarations
Competing interests
A.P.F. is Chief Scientific Officer for Afferent Pharmaceuticals. The other authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 2047 kb)
Supplementary Dataset 1
Supplementary Source Data (XLSX 110 kb)
Rights and permissions
About this article
Cite this article
Pijacka, W., Moraes, D., Ratcliffe, L. et al. Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat Med 22, 1151–1159 (2016). https://doi.org/10.1038/nm.4173
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4173
This article is cited by
-
Carotid body dysregulation contributes to Long COVID symptoms
Communications Medicine (2024)
-
Distribution of P2X3 purinoceptor-immunoreactive sensory nerve endings in the carotid body of Japanese macaque (Macaca fuscata)
Anatomical Science International (2024)
-
Purinergic P2X3 receptors in the carotid body as new therapeutic targets for controlling heart failure
Purinergic Signalling (2024)
-
P2X3 receptor antagonism attenuates the progression of heart failure
Nature Communications (2023)
-
Purinoceptor: a novel target for hypertension
Purinergic Signalling (2023)