Abstract
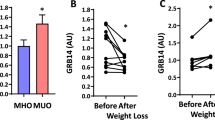
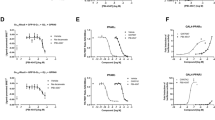
Hepatic insulin resistance is a driving force in the pathogenesis of type 2 diabetes mellitus (T2DM) and is tightly coupled with excessive storage of fat and the ensuing inflammation within the liver1,2,3. There is compelling evidence that activation of the transcription factor nuclear factor-κB (NF-κB) and downstream inflammatory signaling pathways systemically and in the liver are key events in the etiology of hepatic insulin resistance and β-cell dysfunction, although the molecular mechanisms involved are incompletely understood3,4,5,6. We here test the hypothesis that receptor activator of NF-κB ligand (RANKL), a prototypic activator of NF-κB, contributes to this process using both an epidemiological and experimental approach. In the prospective population-based Bruneck Study, a high serum concentration of soluble RANKL emerged as a significant (P < 0.001) and independent risk predictor of T2DM manifestation. In close agreement, systemic or hepatic blockage of RANKL signaling in genetic and nutritional mouse models of T2DM resulted in a marked improvement of hepatic insulin sensitivity and amelioration or even normalization of plasma glucose concentrations and glucose tolerance. Overall, this study provides evidence for a role of RANKL signaling in the pathogenesis of T2DM. If so, translation to the clinic may be feasible given current pharmacological strategies to lower RANKL activity to treat osteoporosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Mazzone, T., Chait, A. & Plutzky, J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet 371, 1800–1809 (2008).
Targher, G., Day, C.P. & Bonora, E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 363, 1341–1350 (2010).
Shoelson, S.E., Herrero, L. & Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 132, 2169–2180 (2007).
Donath, M.Y. et al. Mechanisms of β-cell death in type 2 diabetes. Diabetes 54 (suppl. 2), S108–S113 (2005).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 11, 183–190 (2005).
Arkan, M.C. et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 11, 191–198 (2005).
Anderson, D.M. et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390, 175–179 (1997).
Venuraju, S.M., Yerramasu, A., Corder, R. & Lahiri, A. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J. Am. Coll. Cardiol. 55, 2049–2061 (2010).
Kiechl, S. et al. The osteoprotegerin/RANK/RANKL system: a bone key to vascular disease. Expert Rev. Cardiovasc. Ther. 4, 801–811 (2006).
Lieb, W. et al. Biomarkers of the osteoprotegerin pathway. Clinical correlates, subclinical disease, incident cardiovascular disease, and mortality. Arterioscler. Thromb. Vasc. Biol. 30, 1849–1854 (2010).
Kiechl, S. et al. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 109, 2175–2180 (2004).
Collin-Osdoby, P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ. Res. 95, 1046–1057 (2004).
Semb, A.G. et al. Osteoprotegerin and soluble receptor activator of nuclear factor-κB ligand and risk for coronary events: a nested case-control approach in the prospective EPIC-Norfolk population study 1993–2003. Arterioscler. Thromb. Vasc. Biol. 29, 975–980 (2009).
Kiechl, S. et al. Soluble receptor activator of nuclear factor-κ B ligand and risk for cardiovascular disease. Circulation 116, 385–391 (2007).
Schett, G. et al. Soluble RANKL and risk of nontraumatic fracture. J. Am. Med. Assoc. 291, 1108–1113 (2004).
Terpos, E. et al. Soluble receptor activator of nuclear factor κB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood 102, 1064–1069 (2003).
Ziolkowska, M. et al. High levels of osteoprotegerin and soluble receptor activator of nuclear factor κ B ligand in serum of rheumatoid arthritis patients and their normalization after anti-tumor necrosis factor α treatment. Arthritis Rheum. 46, 1744–1753 (2002).
Moschen, A.R. et al. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut. 54, 479–487 (2005).
Shimizu, H. et al. Angiotensin II accelerates osteoporosis by activating osteoclasts. FASEB J. 22, 2465–2475 (2008).
Nishikawa, M., Nakayama, A., Takahashi, Y., Fukuhara, Y. & Takakura, Y. Reactivation of silenced transgene expression in mouse liver by rapid, large-volume injection of isotonic solution. Hum. Gene Ther. 19, 1009–1020 (2008).
Liu, F., Song, Y. & Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 6, 1258–1266 (1999).
Yang, P.L., Althage, A., Chung, J. & Chisari, F.V. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 99, 13825–13830 (2002).
Jiang, J., Yamato, E. & Miyazaki, J. Intravenous delivery of naked plasmid DNA for in vivo cytokine expression. Biochem. Biophys. Res. Commun. 289, 1088–1092 (2001).
Huang, W. et al. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 59, 347–357 (2010).
Meng, Q. & Cai, D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IκB kinase β (IKKβ)/NF-κB pathway. J. Biol. Chem. 286, 32324–32332 (2011).
Cai, D. & Liu, T. Inflammatory cause of metabolic syndrome via brain stress and NF-κB. Aging (Albany, NY) 4, 98–115 (2012).
Hundal, R.S. et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J. Clin. Invest. 109, 1321–1326 (2002).
Sheng, L. et al. NF-κB–inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nat. Med. 18, 943–949 (2012).
Mauro, C. et al. NF-κB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat. Cell Biol. 13, 1272–1279 (2011).
Secchiero, P. et al. An increased osteoprotegerin serum release characterizes the early onset of diabetes mellitus and may contribute to endothelial cell dysfunction. Am. J. Pathol. 169, 2236–2244 (2006).
Lee, N.K. et al. Endocrine regulation of energy metabolism by the skeleton. Cell 13, 456–469 (2007).
Kanazawa, I. et al. Serum osteocalcin level is positively associated with insulin sensitivity and secretion in patients with type 2 diabetes. Bone 48, 720–725 (2011).
Brennan-Speranza, T.C. et al. Osteoblasts mediate the adverse effects of glucocorticoids on fuel metabolism. J. Clin. Invest. 122, 4172–4189 (2012).
Kong, Y.Y. et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402, 304–309 (1999).
Kindle, L. et al. Human microvascular endothelial cell activation by IL-1 and TNF-α stimulates the adhesion and transendothelial migration of circulating human CD14+ monocytes that develop with RANKL into functional osteoclasts. J. Bone Miner. Res. 21, 193–206 (2006).
Goto, H. et al. Primary human bone marrow adipocytes support TNF-α–induced osteoclast differentiation and function through RANKL expression. Cytokine 56, 662–668 (2011).
Bertolini, D.R. et al. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature 319, 516–518 (1986).
Nathan, D.M. Navigating the choices for diabetes prevention. N. Engl. J. Med. 362, 1533–1535 (2010).
Liu, L., Zhang, C., Hu, Y. & Peng, B. Protective effect of metformin on periapical lesions in rats by decreasing the ratio of receptor activator of nuclear factor κ B ligand/osteoprotegerin. J. Endod. 38, 943–947 (2012).
Mai, Q.G. et al. Metformin stimulates osteoprotegerin and reduces RANKL expression in osteoblasts and ovariectomized rats. J. Cell Biochem. 112, 2902–2909 (2011).
Kiechl, S. et al. Toll-like receptor 4 polymorphisms and atherogenesis. N. Engl. J. Med. 347, 185–192 (2002).
Bonora, E. et al. Population-based incidence rates and risk factors for type 2 diabetes in white individuals: the Bruneck study. Diabetes 53, 1782–1789 (2004).
Gillett, M.J. International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes: Diabetes Care 2009; 32(7): 1327–1334. Clin. Biochem. Rev. 30, 197–200 (2009).
Bonora, E. et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23, 57–63 (2000).
Gutt, M. et al. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res. Clin. Pract. 47, 177–184 (2000).
Sluiter, W.J., Erkelens, D.W., Reitsma, W.D. & Doorenbos, H. Glucose tolerance and insulin release, a mathematical approach I. Assay of the β-cell response after oral glucose loading. Diabetes 25, 241–244 (1976).
D'Agostino, R.B. et al. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat. Med. 9, 1501–1515 (1990).
Stewart, S.A. et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9, 493–501 (2003).
Furuhashi, M. et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid–binding protein aP2. Nature 447, 959–965 (2007).
Jornayvaz, F.R. et al. A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am. J. Physiol. Endocrinol. Metab. 299, E808–E815 (2010).
Wang, L. et al. Peripheral disruption of the Grb10 gene enhances insulin signaling and sensitivity in vivo. Mol. Cell Biol. 27, 6497–6505 (2007).
Giaccari, A. & Rossetti, L. Predominant role of gluconeogenesis in the hepatic glycogen repletion of diabetic rats. J. Clin. Invest. 89, 36–45 (1992).
Giaccari, A. et al. In vivo effects of glucosamine on insulin secretion and insulin sensitivity in the rat: possible relevance to the maladaptive responses to chronic hyperglycaemia. Diabetologia 38, 518–524 (1995).
Muse, E.D. et al. Role of resistin in diet-induced hepatic insulin resistance. J. Clin. Invest. 114, 232–239 (2004).
Bonora, E. et al. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J. Clin. Endocrinol. Metab. 68, 374–378 (1989).
Bligh, E.G. & Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Carr, T.P., Andresen, C.J. & Rudel, L.L. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin. Biochem. 26, 39–42 (1993).
Acknowledgements
This study was supported by the Deutsche Forschungsgemeinschaft (SPP1468-IMMUNOBONE to G.S.), the Bundesministerium für Bildung und Forschung (Bundesministerium für Bildung und Forschung project ANCYLOSS to G.S.), the European Union (Masterswitch to G.S.), the Innovative Medicines Initiative funded project BTCure (to G.S.), the National Institute of Diabetes and Digestive and Kidney Diseases (K24 DK080140 to J.B.M.), the Genomics of Lipid-associated Disorders of the Austrian Genome Research Programme GEN-AU (to F.K.), the 'Pustertaler Verein zur Prävention von Herz- und Hirngefässerkrankungen', the 'Gesundheitsbetrieb Bruneck' and the 'Assessorat für Gesundheitswesen, Familie und Soziales, Bolzano'. G.M. is recipient of the Umberto Di Mario Prize by the Società Italiana di Diabetologia 2011. A.G. was supported by grants from Fondazione Don Gnocchi, Università Cattolica del Sacro Cuore (Fondi Ateneo Linea D.3.2 Sindrome Metabolica) and the Italian Ministry of Education, University and Research (PRIN 2010JS3PMZ_011). A.M. and H.T. were supported by the Christian Doppler Research Society. E.B. is recipient of grants from the Italian Ministry of Education, University and Research and the University of Verona. J.M.P. holds an advanced European Research Council grant and is supported by the Austrian Academy of Sciences, a Grant by the National Bank Foundation and Era of Hope/US Department of Defense. We thank B. Enrich for technical support.
Author information
Authors and Affiliations
Contributions
S.K., G.S. and J. Willeit had the idea for this research, took responsibility for the epidemiological and experimental design of this work and wrote the manuscript. G.S. designed all analyses for the experiments. S.K. and P.W. performed all analyses of the epidemiological portion of the study and were supported by M.K. J. Willeit is the principle investigator of the Bruneck Study. J. Wittman, A.G., A.B., A.R.M., G.M., G.P.S., T.K., S.W., J.L., D.M., U.B. and H.T. performed the in vitro and in vivo experimental analysis. A.G., G.M. and G.P.S. performed the animal clamp studies and contributed to the interpretation of the data. G.E., A.M. and F.O. participated in data collection, laboratory analyses, fund raising and conception of the Bruneck Study. M.S. and F.K. performed genetic analyses and contributed to the interpretation of these data. E.B. contributed to researching data. E.B. and J.B.M. gave their advice regarding study design and analysis. J.M.P. and M.O. conducted the experiments involving Msk-Rank and Rip-Rank mice. E.B., J.B.M., H.T., A.G., J.M.P. and all other authors made a critical revision of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.M.P. has received funding from Amgen.
Supplementary information
Supplementary Text and Figures
Supplementary Data, Supplementary Tables 1–5 and Supplementary Figures 1–5 (PDF 1336 kb)
Rights and permissions
About this article
Cite this article
Kiechl, S., Wittmann, J., Giaccari, A. et al. Blockade of receptor activator of nuclear factor-κB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med 19, 358–363 (2013). https://doi.org/10.1038/nm.3084
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3084
This article is cited by
-
Diabetes mellitus is a potential risk factor for aseptic loosening around hip and knee arthroplasty
BMC Musculoskeletal Disorders (2023)
-
Effect of denosumab on glucose metabolism in postmenopausal osteoporotic women with prediabetes: a study protocol for a 12-month multicenter, open-label, randomized controlled trial
Trials (2023)
-
Diabetes and Osteoporosis
Indian Journal of Orthopaedics (2023)
-
Angry scientists, angry analysts and angry novelists. Reply to Doi SA and Abdulmajeed J [letter]
Diabetologia (2023)
-
Metabolic Health and Disease: A Role of Osteokines?
Calcified Tissue International (2023)