Abstract
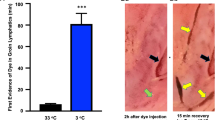
Snake venom toxins first transit the lymphatic system before entering the bloodstream. Ointment containing a nitric oxide donor, which impedes the intrinsic lymphatic pump, prolonged lymph transit time in rats and humans and also increased rat survival time after injection of venom. This pharmacological approach should give snakebite victims more time to obtain medical care and antivenom treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chippaux, J.P. Bull. World Health Organ. 76, 515–524 (1998).
Gilchrist, J. in World Report on Child Injury Prevention (eds. Peden, M. et al.) Chapter 6, Box 6.1 (World Health Organization, Geneva, 2008).
Kasturiratne, A. et al. PLoS Med. 5, e218 (2008).
Barnes, J.M. & Trueta, J. Lancet 237, 623–626 (1941).
Sutherland, S.K., Coulter, A.R. & Harris, R.D. Wilderness Environ. Med. 16, 164–167 (2005).
Anker, R.L., Straffon, W.G. & Loiselle, D.S. Med. J. Aust. 1, 103 (1982).
Hack, J.B. et al. J. Med. Toxicol. 6, 207–211 (2010).
Schmid-Schönbein, G.W. Physiol. Rev. 70, 987–1028 (1990).
Aukland, K. Acta Physiol. Scand. 185, 171–180 (2005).
Olszewski, W.L. & Engeset, A. Am. J. Physiol. 239, H775–H783 (1980).
Yokoyama, S. & Ohhashi, T. Am. J. Physiol. 264, H1460–H1464 (1993).
Howarth, D.M., Southee, A.E. & Whyte, I.M. Med. J. Aust. 161, 695–700 (1994).
Marco, A.J. et al. Lab. Anim. 26, 200–205 (1992).
Rivière, G., Choumet, V., Saliou, B., Debray, M. & Bon, C. J. Pharmacol. Exp. Ther. 285, 490–495 (1998).
Krifi, M.N. et al. Toxicon 45, 187–198 (2005).
Canale, E., Isbister, G.K. & Currie, B.J. Emerg. Med. Australas. 21, 184–190 (2009).
Meggs, W.J., Courtney, C., O'Rourke, D. & Brewer, K.L. Clin. Toxicol. (Phila.) 48, 61–63 (2010).
Acknowledgements
We sincerely thank L. Milward and D. Laver for critical evaluation of the manuscript, the National Health and Medical Research Council of Australia and Hunter Medical Research Institute for funding support and J. Weigel from The Australian Reptile Park for the donation of snake venom.
Author information
Authors and Affiliations
Contributions
M.E.S. conducted the human experiments. P.A.T. supervised the human experiments. P.J.D. conducted the rat experiments. G.K.I. provided expertise on rat experiments, supervised assays and assisted in drafting the manuscript and statistical advice. M.A.O. undertook the absorbance assays. I.M.W. provided input on the human experiments. S.A.M. undertook the statistical analysis and figure presentations. D.F.v.H. conceived of and directed the overall project, supervised the rat experiments, analyzed data and drafted the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.F.v.H. has an Australian patent application (no. 2007327538) for the use of nitric oxide donors as a first aid for treatment of venomous bites.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 and Supplementary Methods (PDF 783 kb)
Rights and permissions
About this article
Cite this article
Saul, M., Thomas, P., Dosen, P. et al. A pharmacological approach to first aid treatment for snakebite. Nat Med 17, 809–811 (2011). https://doi.org/10.1038/nm.2382
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2382