Abstract
Deficiency in mevalonate kinase (MVK) causes systemic inflammation. However, the molecular mechanisms linking the mevalonate pathway to inflammation remain obscure. Geranylgeranyl pyrophosphate, a non-sterol intermediate of the mevalonate pathway, is the substrate for protein geranylgeranylation, a protein post-translational modification that is catalyzed by protein geranylgeranyl transferase I (GGTase I). Pyrin is an innate immune sensor that forms an active inflammasome in response to bacterial toxins. Mutations in MEFV (encoding human PYRIN) result in autoinflammatory familial Mediterranean fever syndrome. We found that protein geranylgeranylation enabled Toll-like receptor (TLR)-induced activation of phosphatidylinositol-3-OH kinase (PI(3)K) by promoting the interaction between the small GTPase Kras and the PI(3)K catalytic subunit p110δ. Macrophages that were deficient in GGTase I or p110δ exhibited constitutive release of interleukin 1β that was dependent on MEFV but independent of the NLRP3, AIM2 and NLRC4 inflammasomes. In the absence of protein geranylgeranylation, compromised PI(3)K activity allows an unchecked TLR-induced inflammatory responses and constitutive activation of the Pyrin inflammasome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Goldstein, J.L. & Brown, M.S. Regulation of the mevalonate pathway. Nature 343, 425–430 (1990).
Frenkel, J. et al. Mevalonate kinase deficiency and Dutch type periodic fever. Clin. Exp. Rheumatol. 18, 525–532 (2000).
Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117, 3720–3732 (2011).
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009).
Kastner, D.L., Aksentijevich, I. & Goldbach-Mansky, R. Autoinflammatory disease reloaded: a clinical perspective. Cell 140, 784–790 (2010).
Xu, H. et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 513, 237–241 (2014).
Beurel, E., Michalek, S.M. & Jope, R.S. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 31, 24–31 (2010).
Hazeki, K., Nigorikawa, K. & Hazeki, O. Role of phosphoinositide 3-kinase in innate immunity. Biol. Pharm. Bull. 30, 1617–1623 (2007).
Androulidaki, A. et al. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity 31, 220–231 (2009).
Martin, M., Rehani, K., Jope, R.S. & Michalek, S.M. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 6, 777–784 (2005).
Weichhart, T. et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29, 565–577 (2008).
Weichhart, T. & Säemann, M.D. The PI3K/Akt/mTOR pathway in innate immune cells: emerging therapeutic applications. Ann. Rheum. Dis. 67 (suppl. 3), iii70–iii74 (2008).
Ohtani, M. et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood 112, 635–643 (2008).
Fritsch, R. et al. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell 153, 1050–1063 (2013).
Zhang, F.L. & Casey, P.J. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65, 241–269 (1996).
Khan, O.M. et al. Geranylgeranyltransferase type I (GGTase-I) deficiency hyperactivates macrophages and induces erosive arthritis in mice. J. Clin. Invest. 121, 628–639 (2011).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Aksoy, E. et al. The p110δ isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat. Immunol. 13, 1045–1054 (2012).
Fukao, T. et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat. Immunol. 3, 875–881 (2002).
Luyendyk, J.P. et al. Genetic analysis of the role of the PI3K-Akt pathway in lipopolysaccharide-induced cytokine and tissue factor gene expression in monocytes/macrophages. J. Immunol. 180, 4218–4226 (2008).
Wang, H., Brown, J. & Martin, M. Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine 53, 130–140 (2011).
Okkenhaug, K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu. Rev. Immunol. 31, 675–704 (2013).
Lucas, C.L. et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat. Immunol. 15, 88–97 (2014).
Gupta, S. et al. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell 129, 957–968 (2007).
Maurer-Stroh, S. et al. Towards complete sets of farnesylated and geranylgeranylated proteins. PLoS Comput. Biol. 3, e66 (2007).
Sebti, S.M. & Hamilton, A.D. Farnesyltransferase and geranylgeranyltransferase I inhibitors and cancer therapy: lessons from mechanism and bench-to-bedside translational studies. Oncogene 19, 6584–6593 (2000).
Kinsella, B.T., Erdman, R.A. & Maltese, W.A. Posttranslational modification of Ha-ras p21 by farnesyl versus geranylgeranyl isoprenoids is determined by the COOH-terminal amino acid. Proc. Natl. Acad. Sci. USA 88, 8934–8938 (1991).
Galeotti, C. et al. Efficacy of interleukin-1-targeting drugs in mevalonate kinase deficiency. Rheumatology (Oxford) 51, 1855–1859 (2012).
Rathinam, V.A. et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150, 606–619 (2012).
Papin, S. et al. The tumor necrosis factor alpha-dependent activation of the human mediterranean fever (MEFV) promoter is mediated by a synergistic interaction between C/EBP beta and NF kappaB p65. J. Biol. Chem. 278, 48839–48847 (2003).
Ni, M. et al. B-cell adaptor for PI3K (BCAP) negatively regulates Toll-like receptor signaling through activation of PI3K. Proc. Natl. Acad. Sci. USA 109, 267–272 (2012).
Sarkar, S.N. et al. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat. Struct. Mol. Biol. 11, 1060–1067 (2004).
Wang, H. et al. IFN-beta production by TLR4-stimulated innate immune cells is negatively regulated by GSK3-beta. J. Immunol. 181, 6797–6802 (2008).
Wang, J.T. et al. Glycogen synthase kinase 3 negatively regulates IFN regulatory factor 3 transactivation through phosphorylation at its linker region. Innate Immun. 20, 78–87 (2014).
Hrincius, E.R. et al. Phosphatidylinositol-3-kinase (PI3K) is activated by influenza virus vRNA via the pathogen pattern receptor Rig-I to promote efficient type I interferon production. Cell. Microbiol. 13, 1907–1919 (2011).
Castellano, E. & Downward, J. Role of RAS in the regulation of PI 3-kinase. Curr. Top. Microbiol. Immunol. 346, 143–169 (2010).
Berndt, N., Hamilton, A.D. & Sebti, S.M. Targeting protein prenylation for cancer therapy. Nat. Rev. Cancer 11, 775–791 (2011).
James, G.L., Goldstein, J.L. & Brown, M.S. Polylysine and CVIM sequences of K-RasB dictate specificity of prenylation and confer resistance to benzodiazepine peptidomimetic in vitro. J. Biol. Chem. 270, 6221–6226 (1995).
Lerner, E.C., Qian, Y., Hamilton, A.D. & Sebti, S.M. Disruption of oncogenic K-Ras4B processing and signaling by a potent geranylgeranyltransferase I inhibitor. J. Biol. Chem. 270, 26770–26773 (1995).
Rowell, C.A., Kowalczyk, J.J., Lewis, M.D. & Garcia, A.M. Direct demonstration of geranylgeranylation and farnesylation of Ki-Ras in vivo. J. Biol. Chem. 272, 14093–14097 (1997).
Engelman, J.A., Luo, J. & Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7, 606–619 (2006).
Sjogren, A.K. et al. GGTase-I deficiency reduces tumor formation and improves survival in mice with K-RAS-induced lung cancer. J. Clin. Invest. 117, 1294–1304 (2007).
Jou, S.T. et al. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Mol. Cell. Biol. 22, 8580–8591 (2002).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Roberts, Z.J. et al. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J. Exp. Med. 204, 1559–1569 (2007).
Bonham, K.S. et al. A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell 156, 705–716 (2014).
Acknowledgements
We thank S. Abusneineh for technical support, K. Halmen for lab management, and colleagues in the Golenbock, Fitzgerald and Kastner laboratories for helpful discussions. We also thank M. Birnbaum (University of Pennsylvania) for the Akt1-deficient mice femurs and M. Trombly for editing the manuscript. We are in debt to R. Finberg and E. St. Clair for critical reading of the manuscript. This work was supported in part by funds from the US National Institutes of Health (National Institute of Allergy and Infectious Diseases 1R01AI110695-01A1 to D.W.), an Innovative Research Grant from the Arthritis Foundation (D.W. and E.M.G.), the Swedish Research Council, and the Heart and Lung Foundation (M.B. and M.A.).
Author information
Authors and Affiliations
Contributions
D.W. conceived and designed the study, conducted most of the experiments and drafted the manuscript. A.S.L., C.E.F., M.K.A., M.S., Z.J., C.V.R., J.C.K., S.C., R.M.G., X.Z. and S.D.F. conducted experiments and/or provided technical support. D.M. conducted experiments and provided lab management. G.G. provided animal colony maintenance and performed experiments. K.G. provided Pik3cd−/− mouse femurs and critically read the manuscript. J.J.C. and D.L.K. provided lab space at the US National Institutes of Health and help with human studies. R.H. contributed to helpful discussion. N.S., E.M.G. and K.A.F. reviewed primary data and critically read the manuscript. D.T.G. provided lab space, reviewed primary data and contributed to helpful discussions. M.O.B. provided the Pggt1bfl/flLyz2-Cre mouse strain, reviewed primary data and contributed to the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
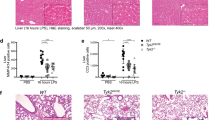
Supplementary Figure 1 Enhanced proinflammatory cytokine production and inhibited IL-10, MIP1α, MIP1α β, MCP-1 and Rantes in Pggt1b-deficient macrophages.
(a) Quantitative RT-PCR analysis of transcript abundance of Il1a, Il1b, TNF, Il6, Il18, Il12a, Ifnb1 and Il10 in Pggt1b+/+ Lyz2-Cre and Pggt1b fl/flLyz2-Cre macrophages stimulated with LPS (200ng/ml) for 0, 2, 4 or 16 hours; (b) Multiplex assay of cytokine production in Pggt1b+/+ Lyz2-Cre and Pggt1b fl/flLyz2-Cre macrophages stimulated with LPS (200ng/ml) for 0, 4 and 24 hours. (*P<0.05, **P<0.001, ***P<0.0001, Two Way ANOVA)
Supplementary Figure 2 Activation of NF-κB, MAP kinases and kinase TBK1 is not impaired in Pggt1b-deficient macrophages.
(a) Immunoblots of IκBα, phospho-IKKβ, phospho-JNK, phospho-p38 and phospho-Erk in the lysate of BMDMs stimulated with LPS (10ng/ml), β-actin and IKKβ are used as loading controls; (b) Immunoblots of phospho-TBK1 in the cell lysate stimulated with LPS (10ng/ml). β-actin and TBK1 are used as loading controls. (c) Flow cytometry analysis of surface TLR4 on BMDMs upon LPS stimulation.
Supplementary Figure 3 Activation of NF-κB, MAP kinases and kinase TBK1 is not impaired in Pggt1b-deficient macrophages.
(a) Immunoblots of IκBα, phospho-IKKβ, phospho-JNK, phospho-p38 and phospho-Erk in the lysate of BMDMs stimulated with LPS (10ng/ml), β-actin and IKKβ are used as loading controls; (b) Immunoblots of phospho-TBK1 in the cell lysate stimulated with LPS (10ng/ml). β-actin and TBK1 are used as loading controls. (c) Flow cytometry analysis of surface TLR4 on BMDMs upon LPS stimulation.
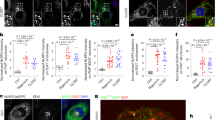
Supplementary Figure 4 Multiple TLR ligands induce inflammasome activation in Pggt1b-deficient BMDMs.
(a) Immunoblots of cleavage of IL-1β in BMDMs stimulated with LPS for different time points; (b) Immunoblots of IL-1β and caspase-1 p20 subunits in the supernatant or the lysate of BMDMs stimulated with ligands to TLR4 (LPS), TLR3 (poly I:C), TLR9 (CpG), TLR1/2 (Pam3CSK4), TLR7 (R848) and Sendai virus (RIG-I); (c) Quantitative reverse-transcripts PCR analysis of the expression of Pggt1b in Pggt1b+/+ Lyz2-Cre, Pggt1bfl/fl Lyz2-Cre or Pggt1bfl/fl Lyz2-Cre BMDMs reconstituted with a retrovirally expressed Pggt1b (Pggt1bfl/fl Lyz2-CreR); (d) Immunoblots of IL-1β and caspase-1 in the supernatant of Pggt1fl/fl Lyz2-Cre macrophages reconstituted with empty pMSCV vector (EV) or that encoding Pggt1b, stimulated with LPS or LPS plus nigericin.
Supplementary Figure 5 LPS-induced inflammasome activation in Pggt1b-deficient BMDMs depends on caspase-1 and Asc but not Nlrp3.
Immunoblots of IL-1β, caspase-1 in the supernatant and lysate of (a) Pggt1bfl/fl Lyz2-Cre, Pggt1bfl/fl Lyz2-Cre Casp-1−/−, Pggt1b+/+ Casp1+/+ or Casp-1−/− BMDMs stimulated with LPS or LPS and Nigericin; (b) Pggt1b+/+ Pycard+/+, Pycard−/−, Pggt1bfl/fl Lyz2-Cre, Pggt1bfl/fl Lyz2-Cre Pycard−/−, or BMDMs stimulated with LPS or LPS and Nigericin; (c) Pggt1b+/+ Nlrp3+/+, Pggt1bfl/fl Lyz2-Cre, Pggt1bfl/fl Lyz2-Cre Nlrp3−/−, or Nlrp3−/− BMDMs stimulated with LPS or LPS and Nigericin.
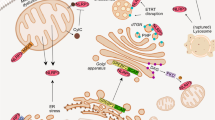
Supplementary Figure 6 Mevalonate pathway controls innate immune response through protein geranylgeranylation.
The mevalonate pathway provides geranylgeranyl pyrophosphate for protein geranylgeranylation. Protein geranylgeranylation regulates TLR-induced PI(3)Kinases activation through geranylgeranylation of Kras. PI(3)Ks and its downstream kinases in turn control the expression of pro- and anti-inflammatory cytokine genes and the Pyrin-encoding Mefv gene in macrophages.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 1 and 2 (PDF 1275 kb)
Rights and permissions
About this article
Cite this article
Akula, M., Shi, M., Jiang, Z. et al. Control of the innate immune response by the mevalonate pathway. Nat Immunol 17, 922–929 (2016). https://doi.org/10.1038/ni.3487
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3487
This article is cited by
-
Tocilizumab for treating mevalonate kinase deficiency and TNF receptor-associated periodic syndrome: a case series and literature review
Pediatric Rheumatology (2024)
-
Pathophysiology, clinical manifestations and current management of IL-1 mediated monogenic systemic autoinflammatory diseases, a literature review
Pediatric Rheumatology (2022)
-
Organellar homeostasis and innate immune sensing
Nature Reviews Immunology (2022)
-
Activation of the plant mevalonate pathway by extracellular ATP
Nature Communications (2022)
-
RHO GTPases: from new partners to complex immune syndromes
Nature Reviews Immunology (2021)