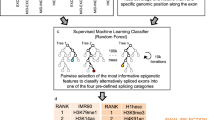
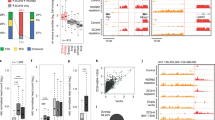
Abstract
The precise splicing of genes confers an enormous transcriptional complexity to the human genome. The majority of gene splicing occurs cotranscriptionally, permitting epigenetic modifications to affect splicing outcomes. Here we show that select exonic regions are demarcated within the three-dimensional structure of the human genome. We identify a subset of exons that exhibit DNase I hypersensitivity and are accompanied by 'phantom' signals in chromatin immunoprecipitation and sequencing (ChIP-seq) that result from cross-linking with proximal promoter- or enhancer-bound factors. The capture of structural features by ChIP-seq is confirmed by chromatin interaction analysis that resolves local intragenic loops that fold exons close to cognate promoters while excluding intervening intronic sequences. These interactions of exons with promoters and enhancers are enriched for alternative splicing events, an effect reflected in cell type–specific periexonic DNase I hypersensitivity patterns. Collectively, our results connect local genome topography, chromatin structure and cis-regulatory landscapes with the generation of human transcriptional complexity by cotranscriptional splicing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pan, Q., Shai, O., Lee, L.J., Frey, B.J. & Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 40, 1413–1415 (2008).
Wang, E.T. et al. Alternative isoform regulation in human tissue transcriptomes. Nature 456, 470–476 (2008).
Maniatis, T. & Tasic, B. Alternative pre-mRNA splicing and proteome expansion in metazoans. Nature 418, 236–243 (2002).
Pozzoli, U. et al. Intron size in mammals: complexity comes to terms with economy. Trends Genet. 23, 20–24 (2007).
Matlin, A.J., Clark, F. & Smith, C.W. Understanding alternative splicing: towards a cellular code. Nat. Rev. Mol. Cell Biol. 6, 386–398 (2005).
Luco, R.F. et al. Regulation of alternative splicing by histone modifications. Science 327, 996–1000 (2010).
Kim, S., Kim, H., Fong, N., Erickson, B. & Bentley, D.L. Pre-mRNA splicing is a determinant of histone H3K36 methylation. Proc. Natl. Acad. Sci. USA 108, 13564–13569 (2011).
Luco, R.F., Allo, M., Schor, I.E., Kornblihtt, A.R. & Misteli, T. Epigenetics in alternative pre-mRNA splicing. Cell 144, 16–26 (2011).
Shukla, S. et al. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 479, 74–79 (2011).
Carrillo Oesterreich, F., Bieberstein, N. & Neugebauer, K.M. Pause locally, splice globally. Trends Cell Biol. 21, 328–335 (2011).
Li, G. et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 148, 84–98 (2012).
Schoenfelder, S., Clay, I. & Fraser, P. The transcriptional interactome: gene expression in 3D. Curr. Opin. Genet. Dev. 20, 127–133 (2010).
Tan-Wong, S.M., French, J.D., Proudfoot, N.J. & Brown, M.A. Dynamic interactions between the promoter and terminator regions of the mammalian BRCA1 gene. Proc. Natl. Acad. Sci. USA 105, 5160–5165 (2008).
Yang, L. et al. ncRNA- and Pc2 methylation–dependent gene relocation between nuclear structures mediates gene activation programs. Cell 147, 773–788 (2011).
Sabo, P.J. et al. Discovery of functional noncoding elements by digital analysis of chromatin structure. Proc. Natl. Acad. Sci. USA 101, 16837–16842 (2004).
Thurman, R.E. et al. The accessible chromatin landscape of the human genome. Nature 489, 75–82 (2012).
Andersson, R., Enroth, S., Rada-Iglesias, A., Wadelius, C. & Komorowski, J. Nucleosomes are well positioned in exons and carry characteristic histone modifications. Genome Res. 19, 1732–1741 (2009).
Tilgner, H. et al. Nucleosome positioning as a determinant of exon recognition. Nat. Struct. Mol. Biol. 16, 996–1001 (2009).
Nahkuri, S., Taft, R.J. & Mattick, J.S. Nucleosomes are preferentially positioned at exons in somatic and sperm cells. Cell Cycle 8, 3420–3424 (2009).
Gerstein, M.B. et al. Architecture of the human regulatory network derived from ENCODE data. Nature 489, 91–100 (2012).
Ernst, J. et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473, 43–49 (2011).
Fullwood, M.J. et al. An oestrogen-receptor-α–bound human chromatin interactome. Nature 462, 58–64 (2009).
Dunham, I. et al. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Buratowski, S. Progression through the RNA polymerase II CTD cycle. Mol. Cell 36, 541–546 (2009).
Cheutin, T. & Cavalli, G. Progressive polycomb assembly on H3K27me3 compartments generates polycomb bodies with developmentally regulated motion. PLoS Genet. 8, e1002465 (2012).
Kassouf, M.T. et al. Genome-wide identification of TAL1's functional targets: insights into its mechanisms of action in primary erythroid cells. Genome Res. 20, 1064–1083 (2010).
Chepelev, I., Wei, G., Wangsa, D., Tang, Q. & Zhao, K. Characterization of genome-wide enhancer-promoter interactions reveals co-expression of interacting genes and modes of higher order chromatin organization. Cell Res. 22, 490–503 (2012).
Ohlsson, R., Lobanenkov, V. & Klenova, E. Does CTCF mediate between nuclear organization and gene expression? Bioessays 32, 37–50 (2010).
Handoko, L. et al. CTCF-mediated functional chromatin interactome in pluripotent cells. Nat. Genet. 43, 630–638 (2011).
Saint-André, V., Batsche, E., Rachez, C. & Muchardt, C. Histone H3 lysine 9 trimethylation and HP1γ favor inclusion of alternative exons. Nat. Struct. Mol. Biol. 18, 337–344 (2011).
Pradeepa, M.M., Sutherland, H.G., Ule, J., Grimes, G.R. & Bickmore, W.A. Psip1/Ledgf p52 binds methylated histone H3K36 and splicing factors and contributes to the regulation of alternative splicing. PLoS Genet. 8, e1002717 (2012).
Tilgner, H. et al. Deep sequencing of subcellular RNA fractions shows splicing to be predominantly co-transcriptional in the human genome but inefficient for lncRNAs. Genome Res. 22, 1616–1625 (2012).
Bhatt, D.M. et al. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell 150, 279–290 (2012).
Djebali, S. et al. Landscape of transcription in human cells. Nature 489, 101–108 (2012).
Harrow, J. et al. GENCODE: producing a reference annotation for ENCODE. Genome Biol. 7 (suppl. 1), S4.1–S4.9 (2006).
Kolasinska-Zwierz, P. et al. Differential chromatin marking of introns and expressed exons by H3K36me3. Nat. Genet. 41, 376–381 (2009).
Anders, S., Reyes, A. & Huber, W. Detecting differential usage of exons from RNA-seq data. Genome Res. 22, 2008–2017 (2012).
Cramer, P., Pesce, C.G., Baralle, F.E. & Kornblihtt, A.R. Functional association between promoter structure and transcript alternative splicing. Proc. Natl. Acad. Sci. USA 94, 11456–11460 (1997).
Kornblihtt, A.R. Promoter usage and alternative splicing. Curr. Opin. Cell Biol. 17, 262–268 (2005).
Schwartz, S. & Ast, G. Chromatin density and splicing destiny: on the cross-talk between chromatin structure and splicing. EMBO J. 29, 1629–1636 (2010).
Deng, W. et al. Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell 149, 1233–1244 (2012).
Razin, S.V. et al. Transcription factories in the context of the nuclear and genome organization. Nucleic Acids Res. 39, 9085–9092 (2011).
Mao, Y.S., Zhang, B. & Spector, D.L. Biogenesis and function of nuclear bodies. Trends Genet. 27, 295–306 (2011).
Melnik, S. et al. The proteomes of transcription factories containing RNA polymerases I, II or III. Nat. Methods 8, 963–968 (2011).
Edelman, L.B. & Fraser, P. Transcription factories: genetic programming in three dimensions. Curr. Opin. Genet. Dev. 22, 110–114 (2012).
Allemand, E., Batsche, E. & Muchardt, C. Splicing, transcription, and chromatin: a menage a trois. Curr. Opin. Genet. Dev. 18, 145–151 (2008).
Moldón, A. et al. Promoter-driven splicing regulation in fission yeast. Nature 455, 997–1000 (2008).
Gerstein, M.B. et al. What is a gene, post-ENCODE? History and updated definition. Genome Res. 17, 669–681 (2007).
Kapranov, P., Willingham, A.T. & Gingeras, T.R. Genome-wide transcription and the implications for genomic organization. Nat. Rev. Genet. 8, 413–423 (2007).
Mercer, T.R. et al. Targeted RNA sequencing reveals the deep complexity of the human transcriptome. Nat. Biotechnol. 30, 99–104 (2012).
Carninci, P. et al. The transcriptional landscape of the mammalian genome. Science 309, 1559–1563 (2005).
Derrien, T. et al. Fast computation and applications of genome mappability. PLoS ONE 7, e30377 (2012).
Li, G. et al. ChIA-PET tool for comprehensive chromatin interaction analysis with paired-end tag sequencing. Genome Biol. 11, R22 (2010).
ENCODE Project Consortium. User's guide to the encyclopedia of DNA elements (ENCODE). PLoS Biol. 9, e1001046 (2011).
Sabo, P.J. et al. Genome-scale mapping of DNase I sensitivity in vivo using tiling DNA microarrays. Nat. Methods 3, 511–518 (2006).
de Hoon, M.J., Imoto, S., Nolan, J. & Miyano, S. Open source clustering software. Bioinformatics 20, 1453–1454 (2004).
Matys, V. et al. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 34, D108–D110 (2006).
Grant, C.E., Bailey, T.L. & Noble, W.S. FIMO: scanning for occurrences of a given motif. Bioinformatics 27, 1017–1018 (2011).
Tan-Wong, S.M., Wijayatilake, H.D. & Proudfoot, N.J. Gene loops function to maintain transcriptional memory through interaction with the nuclear pore complex. Genes Dev. 23, 2610–2624 (2009).
Acknowledgements
The authors would like to thank the following funding sources: the Australian National Health and Medical Research Council (Australia Fellowships 631668 to J.S.M., T.R.M. and M.B.C. and 631381 and 1021731 to S.L.E.); the Queensland State Government (National and International Research Alliance Program to L.K.N.); the National Breast Cancer Foundation Australia (to S.L.E.); the National Human Genome Research Institute (NHGRI; ENCODE grant HG004456 to Y.R., G.L. and K.S.S.); and the US National Institutes of Health (NHGRI ENCODE grants U54HG004592 and U54HG007599 to J.A.S.).
Author information
Authors and Affiliations
Contributions
T.R.M., G.L. and S.J.N. performed bioinformatic analysis. S.L.E. performed 3C analysis. G.L., K.S.S. and Y.R. performed ChIA-PET analysis. A.B.S., H.W., S.J. and R.S. performed native ChIP-seq and whole-genome sequencing. T.R.M., S.J.N., M.B.C., L.K.N., Y.R., J.S.M. and J.A.S. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Table 1 (PDF 1729 kb)
Rights and permissions
About this article
Cite this article
Mercer, T., Edwards, S., Clark, M. et al. DNase I–hypersensitive exons colocalize with promoters and distal regulatory elements. Nat Genet 45, 852–859 (2013). https://doi.org/10.1038/ng.2677
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2677
This article is cited by
-
Evidence for the role of transcription factors in the co-transcriptional regulation of intron retention
Genome Biology (2023)
-
Construction and characterization of EGFP reporter plasmid harboring putative human RAX promoter for in vitro monitoring of retinal progenitor cells identity
BMC Molecular and Cell Biology (2021)
-
High frequency of intron retention and clustered H3K4me3-marked nucleosomes in short first introns of human long non-coding RNAs
Epigenetics & Chromatin (2021)
-
The landscape of somatic mutation in cerebral cortex of autistic and neurotypical individuals revealed by ultra-deep whole-genome sequencing
Nature Neuroscience (2021)
-
Integrative prediction of gene expression with chromatin accessibility and conformation data
Epigenetics & Chromatin (2020)