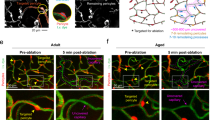
Abstract
Pericytes and smooth muscle cells are integral components of the brain microvasculature. However, no techniques exist to unambiguously identify these cell types, greatly limiting their investigation in vivo. Here we show that the fluorescent Nissl dye NeuroTrace 500/525 labels brain pericytes with specificity, allowing high-resolution optical imaging in the live mouse. We demonstrate that capillary pericytes are a population of mural cells with distinct morphological, molecular and functional features that do not overlap with precapillary or arteriolar smooth muscle actin-expressing cells. The remarkable specificity for dye uptake suggests that pericytes have molecular transport mechanisms not present in other brain cells. We demonstrate feasibility of longitudinal pericyte imaging during microvascular development and aging and in models of brain ischemia and Alzheimer's disease. The ability to easily label pericytes in any mouse model opens the possibility of a broad range of investigations of mural cells in vascular development, neurovascular coupling and neuropathology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Rouget, C. Note sur le développement de la tunique contractile des vaisseaux. Comptes rendus des Séances de l′Acad. des Sciences 79, 559 (1874).
Armulik, A., Genové, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 (2011).
Iadecola, C. & Nedergaard, M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 10, 1369–1376 (2007).
Daneman, R., Zhou, L., Kebede, A.A. & Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010).
Armulik, A. et al. Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010).
Shih, A.Y. et al. Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture. Microcirculation 22, 204–218 (2015).
Hill, R.A. et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 87, 95–110 (2015).
Winkler, E.A., Bell, R.D. & Zlokovic, B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398–1405 (2011).
Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 5, 347–360 (2004).
Hall, C.N. et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60 (2014).
Fernández-Klett, F., Offenhauser, N., Dirnagl, U., Priller, J. & Lindauer, U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc. Natl. Acad. Sci. USA 107, 22290–22295 (2010).
Yemisci, M. et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 15, 1031–1037 (2009).
Hartmann, D.A. et al. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2, 041402 (2015).
Wei, H.S. et al. Erythrocytes are oxygen-sensing regulators of the cerebral microcirculation. Neuron 91, 851–862 (2016).
Vates, G.E., Takano, T., Zlokovic, B. & Nedergaard, M. Pericyte constriction after stroke: the jury is still out. Nat. Med. 16, 959–960 (2010).
Sweeney, M.D., Ayyadurai, S. & Zlokovic, B.V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 19, 771–783 (2016).
Attwell, D., Mishra, A., Hall, C.N., O'Farrell, F.M. & Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. 36, 451–455 (2016).
Quinn, B., Toga, A.W., Motamed, S. & Merlic, C.A. Fluoro Nissl green: a novel fluorescent counterstain for neuroanatomy. Neurosci. Lett. 184, 169–172 (1995).
Nimmerjahn, A., Kirchhoff, F., Kerr, J.N.D. & Helmchen, F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat. Methods 1, 31–37 (2004).
Hill, R.A. & Grutzendler, J. In vivo imaging of oligodendrocytes with sulforhodamine 101. Nat. Methods 11, 1081–1082 (2014).
Cuttler, A.S. et al. Characterization of Pdgfrb-Cre transgenic mice reveals reduction of ROSA26 reporter activity in remodeling arteries. Genesis 49, 673–680 (2011).
Zhu, X., Bergles, D.E. & Nishiyama, A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 135, 145–157 (2008).
Zhu, X. et al. Age-dependent fate and lineage restriction of single NG2 cells. Development 138, 745–753 (2011).
Meisslitzer-Ruppitsch, C., Röhrl, C., Neumüller, J., Pavelka, M. & Ellinger, A. Photooxidation technology for correlated light and electron microscopy. J. Microsc. 235, 322–335 (2009).
Meiblitzer-Ruppitsch, C. et al. Electron microscopic visualization of fluorescent signals in cellular compartments and organelles by means of DAB-photoconversion. Histochem. Cell Biol. 130, 407–419 (2008).
Morris, A.W.J. et al. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 131, 725–736 (2016).
Armstrong, J.J., Larina, I.V., Dickinson, M.E., Zimmer, W.E. & Hirschi, K.K. Characterization of bacterial artificial chromosome transgenic mice expressing mCherry fluorescent protein substituted for the murine smooth muscle alpha-actin gene. Genesis 48, 457–463 (2010).
Shen, Z., Lu, Z., Chhatbar, P.Y., O'Herron, P. & Kara, P. An artery-specific fluorescent dye for studying neurovascular coupling. Nat. Methods 9, 273–276 (2012).
Harb, R., Whiteus, C., Freitas, C. & Grutzendler, J. In vivo imaging of cerebral microvascular plasticity from birth to death. J. Cereb. Blood Flow Metab. 33, 146–156 (2013).
Gerhardt, H. & Betsholtz, C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 314, 15–23 (2003).
Hughes, S. & Chan-Ling, T. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest. Ophthalmol. Vis. Sci. 45, 2795–2806 (2004).
Murphy, T.H., Li, P., Betts, K. & Liu, R. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J. Neurosci. 28, 1756–1772 (2008).
Drew, P.J., Shih, A.Y. & Kleinfeld, D. Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity. Proc. Natl. Acad. Sci. USA 108, 8473–8478 (2011).
Simard, M., Arcuino, G., Takano, T., Liu, Q.S. & Nedergaard, M. Signaling at the gliovascular interface. J. Neurosci. 23, 9254–9262 (2003).
Borysova, L., Wray, S., Eisner, D.A. & Burdyga, T. How calcium signals in myocytes and pericytes are integrated across in situ microvascular networks and control microvascular tone. Cell Calcium 54, 163–174 (2013).
Cuevas, P. et al. Pericyte endothelial gap junctions in human cerebral capillaries. Anat. Embryol. (Berl.) 170, 155–159 (1984).
Bell, R.D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Oakley, H. et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 26, 10129–10140 (2006).
Holtmaat, A. et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4, 1128–1144 (2009).
Whiteus, C., Freitas, C. & Grutzendler, J. Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature 505, 407–411 (2014).
Sano, H. et al. Study on PDGF receptor β pathway in glomerular formation in neonate mice. Ann. NY Acad. Sci. 947, 303–305 (2001).
Hill, R.A., Natsume, R., Sakimura, K. & Nishiyama, A. NG2 cells are uniformly distributed and NG2 is not required for barrel formation in the somatosensory cortex. Mol. Cell. Neurosci. 46, 689–698 (2011).
Acknowledgements
We thank K. Hirschi (Yale University) for sharing SMA-mCherry mice, V. Lindner (Maine Medical Center Research Institute) for sharing Pdgfrb-cre mice, A. Nishiyama (University of Connecticut) for sharing NG2-cre mice and R. Vassar (Northwestern University) for sharing 5xFAD mice. We thank X. Liu and M. Graham at the biological electron microscopy facility core (Yale School of Medicine) for assistance with sample preparation and imaging. This work was supported by the following grants from the National Institutes of Health: R21NS087511, R21NS088411, R01NS0889734 and R21AG048181 to J.G. and F32NS090820 to R.A.H.
Author information
Authors and Affiliations
Contributions
E.C.D., R.A.H. and J.G. made the initial observation and designed all experiments. E.C.D. and R.A.H. performed the morphological, developmental and functional characterization of dye-labeled cells. L.T. conducted Alzheimer's mouse model characterization and prepared tissue for electron microscopy. K.N.M. performed fixed tissue quantification and surgeries for carotid occlusion experiments. R.A.H. and J.G. wrote the manuscript with input from all authors. J.G. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Pdgfrb-cre:Tomato transgenic mice exhibit complete labeling of vascular mural cells that are colabeled with Pdgfrb and NG2 antibodies.
a) Confocal fluorescence images captured from the cortex of Pdgfrb-cre:Tomato transgenic mice stained with an antibody against Pdgfrb (Pdgfrb Ab) showing complete overlap between Tomato expressing mural cells (arrowheads) and antibody staining.
(b) Confocal fluorescence images captured from the cortex of Pdgfrb-cre:Tomato transgenic mice stained with an antibody against NG2 (NG2 Ab) showing expected NG2 expression by mural cells (arrowheads) and also by NG2-glia and processes in the parenchyma (arrows).
Supplementary Figure 2 NeuroTrace-labeled pericytes are embedded in the basal lamina.
(a) Confocal fluorescence images captured from the cortex of Pdgfrb-cre:Tomato transgenic mice stained with collagen IV antibody showing detection of the double layer of the basal lamina completely surrounding capillary pericyte previously labeled with NeuroTrace dye. Single pericytes labeled with NeuroTrace and Tomato were imaged immediately after tissue sectioning but before immunostaining and then reimaged after immunostaining for collagen IV confirming that NeuroTrace labels capillary pericytes (n=11 cells). Top panel is the same cell shown in Figure 4c top only a single z plane. All images are single z-plane.
Supplementary Figure 3 Intracerebral injection results in rapid, exclusive labeling of capillary pericytes
(a-b) Schematic, in vivo images and time-lapse sequence showing intracerebral injection of NeuroTrace resulting in exclusive pericyte labeling minutes after dye injection (arrows). Location of injection site is indicated by drawing of the glass micropipette within the image.
(c) NeuroTrace fluorescence intensity measurements during intracerebral injection of NeuroTrace dye at 4 regions of interest (ROIs) showing specific targeting of capillary pericytes indicated by ROIs 2 and 4.
Supplementary Figure 4 Microvascular pathology during cerebral ischemia
(a) Schematic showing timeline and approach for in vivo imaging during transient cerebral ischemia.
(b-d) In vivo time-lapse sequences captured from the cerebral cortex showing collapse and reperfusion of SMC-covered (arrows) (SMA-mCherry+) but no change in diameter in pericyte-covered (NeuroTrace+) vessels.
(e) Percent changes in diameter from baseline in SMC and pericyte covered vessels showing significant vessel collapse/constriction during ischemia only on SMC-covered vessels (pericyte covered: 28 vessel locations, SMC covered: 23 vessel locations from n=3 mice, unpaired two-tailed student’s t-test p values as indicated during: t=6.244279 df=4, after: t=1.104133 df=4).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 1007 kb)
NeuroTrace labeling in the live mouse cortex.
Video shows a z-stack of NeuroTrace labeling in the cortex of a live mouse with intravascular dye used to visualize the cortical blood vessels. Depth into the cortex is indicated in upper right corner, with NeuroTrace labeling reaching up to 400 μm into the tissue. (MP4 6873 kb)
Exclusive pericyte labeling after intracerebral NeuroTrace injection.
Video shows an in vivo time-lapse sequence captured during consecutive injections of NeuroTrace into the cerebral cortex, showing exclusive labeling of pericytes within minutes after parenchymal injection. Injections are indicated as green rectangles in upper right corner and time is indicated in seconds. (MP4 26634 kb)
Rights and permissions
About this article
Cite this article
Damisah, E., Hill, R., Tong, L. et al. A fluoro-Nissl dye identifies pericytes as distinct vascular mural cells during in vivo brain imaging. Nat Neurosci 20, 1023–1032 (2017). https://doi.org/10.1038/nn.4564
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4564
This article is cited by
-
The role of cardiac pericytes in health and disease: therapeutic targets for myocardial infarction
Nature Reviews Cardiology (2024)
-
Subcellular analysis of blood-brain barrier function by micro-impalement of vessels in acute brain slices
Nature Communications (2023)
-
Fluorescence-amplified nanocrystals in the second near-infrared window for in vivo real-time dynamic multiplexed imaging
Nature Nanotechnology (2023)
-
The Provenance, Providence, and Position of Endothelial Cells in Injured Spinal Cord Vascular Pathology
Cellular and Molecular Neurobiology (2023)
-
Targeting Pericytes for Functional Recovery in Ischemic Stroke
NeuroMolecular Medicine (2023)