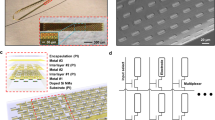
Abstract
Arrays of electrodes for recording and stimulating the brain are used throughout clinical medicine and basic neuroscience research, yet are unable to sample large areas of the brain while maintaining high spatial resolution because of the need to individually wire each passive sensor at the electrode-tissue interface. To overcome this constraint, we developed new devices that integrate ultrathin and flexible silicon nanomembrane transistors into the electrode array, enabling new dense arrays of thousands of amplified and multiplexed sensors that are connected using fewer wires. We used this system to record spatial properties of cat brain activity in vivo, including sleep spindles, single-trial visual evoked responses and electrographic seizures. We found that seizures may manifest as recurrent spiral waves that propagate in the neocortex. The developments reported here herald a new generation of diagnostic and therapeutic brain-machine interface devices.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Freeman, W.J. Spatial spectral analysis of human electrocorticograms including the alpha and gamma bands. J. Neurosci. Methods 95, 111–121 (2000).
Kellis, S.S., House, P.A., Thomson, K.E., Brown, R. & Greger, B. Human neocortical electrical activity recorded on nonpenetrating microwire arrays: applicability for neuroprostheses. Neurosurg. Focus 27, E9 (2009).
Kellis, S. et al. Decoding spoken words using local field potentials recorded from the cortical surface. J. Neural Eng. 7, 056007 (2010).
Kitzmiller, J.P. et al. Micro-field evoked potentials recorded from the porcine sub-dural cortical surface utilizing a microelectrode array. J. Neurosci. Methods 162, 155–161 (2007).
Schevon, C.A. et al. Microphysiology of epileptiform activity in human neocortex. J. Clin. Neurophysiol. 25, 321–330 (2008).
Stead, M. et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain 133, 2789–2797 (2010).
Amunts, K., Malikovic, A., Mohlberg, H., Schormann, T. & Zilles, K. Brodmann's areas 17 and 18 brought into stereotaxic space-where and how variable? Neuroimage 11, 66–84 (2000).
Branco, D.M. et al. Functional variability of the human cortical motor map: electrical stimulation findings in perirolandic epilepsy surgery. J. Clin. Neurophysiol. 20, 17–25 (2003).
Fox, P.T. et al. Location-probability profiles for the mouth region of human primary motor-sensory cortex: model and validation. Neuroimage 13, 196–209 (2001).
Van Essen, D.C., Newsome, W.T. & Maunsell, J.H. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 24, 429–448 (1984).
Campbell, P.K., Jones, K.E., Huber, R.J., Horch, K.W. & Normann, R.A. A silicon-based, three-dimensional neural interface: manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 38, 758–768 (1991).
Hochberg, L.R. et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442, 164–171 (2006).
Ryu, S.I. & Shenoy, K.V. Human cortical prostheses: lost in translation? Neurosurg. Focus 27, E5 (2009).
Polikov, V.S., Tresco, P.A. & Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148, 1–18 (2005).
Schmidt, S., Horch, K. & Normann, R. Biocompatibility of silicon-based electrode arrays implanted in feline cortical tissue. J. Biomed. Mater. Res. 27, 1393–1399 (1993).
Griffith, R.W. & Humphrey, D.R. Long-term gliosis around chronically implanted platinum electrodes in the Rhesus macaque motor cortex. Neurosci. Lett. 406, 81–86 (2006).
Margalit, E. Visual and electrical evoked response recorded from subdural electrodes implanted above the visual cortex in normal dogs under two methods of anesthesia. J. Neurosci. Methods 123, 129–137 (2003).
Chao, Z.C., Nagasaka, Y. & Fujii, N. Long-term asynchronous decoding of arm motion using electrocorticographic signals in monkeys. Front. Neuroeng. 3, 3 (2010).
Yeager, J.D., Phillips, D.J., Rector, D.M. & Bahr, D.F. Characterization of flexible ECoG electrode arrays for chronic recording in awake rats. J. Neurosci. Methods 173, 279–285 (2008).
Andersen, R.A., Musallam, S. & Pesaran, B. Selecting the signals for a brain-machine interface. Curr. Opin. Neurobiol. 14, 720–726 (2004).
Mehring, C. et al. Inference of hand movements from local field potentials in monkey motor cortex. Nat. Neurosci. 6, 1253–1254 (2003).
Brunner, P., Ritaccio, A.L., Emrich, J.F., Bischof, H. & Schalk, G. Rapid communication with a “P300” matrix speller using electrocorticographic signals (ECoG). Front. Neurosci. 5, 5 (2011).
Streetman, B.G. & Banerjee, S.K. Solid State Electronic Devices (Pearson, 1981).
Viventi, J. et al. A conformal, bio-interfaced class of silicon electronics for mapping cardiac electrophysiology. Sci. Transl. Med. 2, 24ra22 (2010).
Yanagisawa, T. et al. Neural decoding using gyral and intrasulcal electrocorticograms. Neuroimage 45, 1099–1106 (2009).
Besson, P., Andermann, F., Dubeau, F. & Bernasconi, A. Small focal cortical dysplasia lesions are located at the bottom of a deep sulcus. Brain 131, 3246–3255 (2008).
Stieglitz, T. Flexible biomedical microdevices with double-sided electrode arrangements for neural applications. Sens. Actuators A Phys. 90, 203–211 (2001).
Stieglitz, T. Flexible BIOMEMS with electrode arrangements on front and back side as key component in neural prostheses and biohybrid systems. Sens. Actuators B Chem. 83, 8–14 (2002).
Thompson, S.E. et al. A 90-nm logic technology featuring strained-silicon. IEEE Trans. Electron. Dev. 51, 1790–1797 (2004).
Kim, D.-H. et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 9, 511–517 (2010).
Padnick, L.B. & Linsenmeier, R.A. Properties of the flash visual evoked potential recorded in the cat primary visual cortex. Vision Res. 39, 2833–2840 (1999).
Tusa, R.J., Rosenquist, A.C. & Palmer, L.A. Retinotopic organization of areas 18 and 19 in the cat. J. Comp. Neurol. 185, 657–678 (1979).
Hinton, G.E. & Salakhutdinov, R.R. Reducing the dimensionality of data with neural networks. Science 313, 504–507 (2006).
Larochelle, H., Erhan, D., Courville, A., Bergstra, J. & Bengio, Y. An empirical evaluation of deep architectures on problems with many factors of variation. Proc. Int. Conf. Mach. Learn. 473–480 (2007).
Anderson, W.S., Kudela, P., Cho, J., Bergey, G.K. & Franaszczuk, P.J. Studies of stimulus parameters for seizure disruption using neural network simulations. Biol. Cybern. 97, 173–194 (2007).
Witkowski, F.X. et al. Spatiotemporal evolution of ventricular fibrillation. Nature 392, 78–82 (1998).
Prechtl, J.C., Cohen, L.B., Pesaran, B., Mitra, P.P. & Kleinfeld, D. Visual stimuli induce waves of electrical activity in turtle cortex. Proc. Natl. Acad. Sci. USA 94, 7621–7626 (1997).
Huang, X. et al. Spiral waves in disinhibited mammalian neocortex. J. Neurosci. 24, 9897–9902 (2004).
Huang, X. et al. Spiral wave dynamics in neocortex. Neuron 68, 978–990 (2010).
Hastie, T., Tibshirani, R. & Friedman, J. The Elements of Statistical Learning. (Springer-Verlag, New York, 2001).
Tibshirani, R., Walther, G. & Hastie, T. Estimating the number of clusters in a data set via the gap statistic. J. R. Stat. Soc. Series B Stat. Methodol. 63, 411–423 (2001).
Blanco, J.A. et al. Unsupervised classification of high-frequency oscillations in human neocortical epilepsy and control patients. J. Neurophysiol. 104, 2900–2912 (2010).
Paullet, J.E. & Ermentrout, G.B. Stable rotating waves in two-dimensional discrete active media. SIAM J. Appl. Math. 54, 1720 (1994).
Gais, S., Mölle, M., Helms, K. & Born, J. Learning-dependent increases in sleep spindle density. J. Neurosci. 22, 6830–6834 (2002).
Tamminen, J., Payne, J.D., Stickgold, R., Wamsley, E.J. & Gaskell, M.G. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J. Neurosci. 30, 14356–14360 (2010).
Sekitani, T. et al. A large-area wireless power-transmission sheet using printed organic transistors and plastic MEMS switches. Nat. Mater. 6, 413–417 (2007).
Llinás, R.R., Ribary, U., Jeanmonod, D., Kronberg, E. & Mitra, P.P. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. USA 96, 15222–15227 (1999).
Cardin, J.A., Palmer, L.A. & Contreras, D. Stimulus feature selectivity in excitatory and inhibitory neurons in primary visual cortex. J. Neurosci. 27, 10333 (2007).
Cardin, J.A., Palmer, L.A. & Contreras, D. Cellular mechanisms underlying stimulus-dependent gain modulation in primary visual cortex neurons in vivo. Neuron 59, 150–160 (2008).
Taylor, Z. & Miller, K. Reassessment of brain elasticity for analysis of biomechanisms of hydrocephalus. J. Biomech. 37, 1263–1269 (2004).
Acknowledgements
This work was supported by the National Science Foundation (grant DMI-0328162) and the US Department of Energy, Division of Materials Sciences (Award No. DE-FG02-07ER46471), through the Materials Research Laboratory and Center for Microanalysis of Materials (DE-FG02-07ER46453) at the University of Illinois at Urbana-Champaign. J.A.R. acknowledges a National Security Science and Engineering Faculty Fellowship. Work at the University of Pennsylvania was supported by grants from the US National Institutes of Health (National Institute of Neurological Disorders and Stroke RO1-NS041811 and RO1-NS48598), the Julie's Hope Award from the Citizens United for Research in Epilepsy, and the Dr. Michel and Mrs. Anna Mirowski Discovery Fund for Epilepsy Research. J.V. received a Ruth L. Kirschstein National Research Service Award (2T32HL007954) from the US National Institutes of Health, National Heart, Lung and Blood Institute.
Author information
Authors and Affiliations
Contributions
J.V., D.-H.K., L.V., A.E.A., V.R.T., L.P., J.V.S., D.C., J.A.R. and B.L. designed the experiments. J.V., D.-H.K., L.V., J.A.B., Y.-S.K., S.-W.H., A.C.V., D.F.W., K.D., E.S.F., C.E.G., R.Y., J.W. and J.X. performed the experiments and analysis. J.V., D.-H.K., L.V., J.A.B., E.S.F., Y.H., D.C., J.A.R. and B.L. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–27 (PDF 11216 kb)
Supplementary Movie 1
Movie of a short electrographic seizure showing numerous complicated spatial patterns, including clockwise and counterclockwise spiral waves. The voltage for all 360 channels is plotted as a color map in the top of the frame, while the average of all 360 electrodes is plotted at the bottom of the frame with a vertical bar indicating the position in time for reference. The movie is presented ~18× slower than real-time. (MPG 29888 kb)
Rights and permissions
About this article
Cite this article
Viventi, J., Kim, DH., Vigeland, L. et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci 14, 1599–1605 (2011). https://doi.org/10.1038/nn.2973
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2973
This article is cited by
-
Flexible switch matrix addressable electrode arrays with organic electrochemical transistor and pn diode technology
Nature Communications (2024)
-
Fully bioresorbable hybrid opto-electronic neural implant system for simultaneous electrophysiological recording and optogenetic stimulation
Nature Communications (2024)
-
Shape-changing electrode array for minimally invasive large-scale intracranial brain activity mapping
Nature Communications (2024)
-
Solar manipulations of perpendicular magnetic anisotropy for flexible spintronics
Frontiers of Physics (2024)
-
Unveiling the mechanism of remote epitaxy of crystalline semiconductors on 2D materials-coated substrates
Nano Convergence (2023)