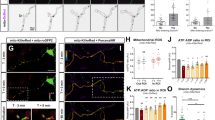
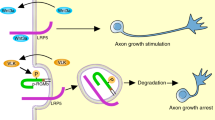
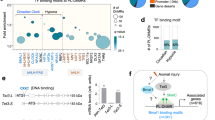
Abstract
The refinement of neural circuits during development depends on a dynamic process of branching of axons and dendrites that leads to synapse formation and connectivity. The neurotrophin brain-derived neurotrophic factor (BDNF) is essential for the outgrowth and activity-dependent remodeling of axonal arbors in vivo. However, the mechanisms that translate extracellular signals into the formation of axonal branches are incompletely understood. We found that MAP kinase phosphatase-1 (MKP-1) controls axon branching. MKP-1 expression induced by BDNF signaling caused spatiotemporal deactivation of c-jun N-terminal kinase (JNK), which reduced the phosphorylation of JNK substrates that destabilize microtubules. Indeed, neurons from mkp-1 null mice could not produce axon branches in response to BDNF. Our results identify a signaling mechanism that regulates axonal branching and provide a framework for studying the molecular mechanisms of innervation and axonal remodeling under normal and pathological conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cohen-Cory, S. & Fraser, S.E. Effects of brain-derived neurotrophic factor on optic axon branching and remodeling in vivo. Nature 378, 192–196 (1995).
McAllister, A.K., Katz, L.C. & Lo, D.C. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 22, 295–318 (1999).
Marshak, S., Nikolakopoulou, A.M., Dirks, R., Martens, G.J. & Cohen-Cory, S. Cell-autonomous TrkB signaling in presynaptic retinal ganglion cells mediates axon arbor growth and synapse maturation during the establishment of retinotectal synaptic connectivity. J. Neurosci. 27, 2444–2456 (2007).
Huang, J.K., Dorey, K., Ishibashi, S. & Amaya, E. BDNF promotes target innervation of Xenopus mandibular trigeminal axons in vivo. BMC Dev. Biol. 7, 59 (2007).
Danzer, S.C., Crooks, K.R., Lo, D.C. & McNamara, J.O. Increased expression of brain-derived neurotrophic factor induces formation of basal dendrites and axonal branching in dentate granule cells in hippocampal explant cultures. J. Neurosci. 22, 9754–9763 (2002).
Martínez, A. et al. TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J. Neurosci. 18, 7336–7350 (1998).
Li, Z., Van Aelst, L. & Cline, H.T. Rho GTPases regulate distinct aspects of dendritic arbor growth in Xenopus central neurons in vivo. Nat. Neurosci. 3, 217–225 (2000).
Hutchins, B.I. & Kalil, K. Differential outgrowth of axons and their branches is regulated by localized calcium transients. J. Neurosci. 28, 143–153 (2008).
Gerecke, K.M., Wyss, J.M. & Carroll, S.L. Neuregulin-1β induces neurite extension and arborization in cultured hippocampal neurons. Mol. Cell. Neurosci. 27, 379–393 (2004).
Marshall, C.J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal–regulated kinase activation. Cell 80, 179–185 (1995).
Chao, M.V. Growth factor signaling: where is the specificity? Cell 68, 995–997 (1992).
Boutros, T., Chevet, E. & Metrakos, P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death and cancer. Pharmacol. Rev. 60, 261–310 (2008).
Keyse, S.M. & Emslie, E.A. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature 359, 644–647 (1992).
Davis, S., Vanhoutte, P., Pages, C., Caboche, J. & Laroche, S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element–binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J. Neurosci. 20, 4563–4572 (2000).
Doi, M. et al. Light-inducible and clock-controlled expression of MAP kinase phosphatase 1 in mouse central pacemaker neurons. J. Biol. Rhythms 22, 127–139 (2007).
Carrasco, D. & Bravo, R. Expression of the nontransmembrane tyrosine phosphatase gene erp during mouse organogenesis. Cell Growth Differ. 4, 849–859 (1993).
Maisonpierre, P.C. et al. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron 5, 501–509 (1990).
Langevin, L.M. et al. Validating in utero electroporation for the rapid analysis of gene regulatory elements in the murine telencephalon. Dev. Dyn. 236, 1273–1286 (2007).
Owens, D.M. & Keyse, S.M. Differential regulation of MAP kinase signaling by dual-specificity protein phosphatases. Oncogene 26, 3203–3213 (2007).
Oliva, A.A. Jr., Atkins, C.M., Copenagle, L. & Banker, G.A. Activated c-Jun N-terminal kinase is required for axon formation. J. Neurosci. 26, 9462–9470 (2006).
Slack, D.N., Seternes, O.M., Gabrielsen, M. & Keyse, S.M. Distinct binding determinants for ERK2/p38α and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. J. Biol. Chem. 276, 16491–16500 (2001).
Camps, M. et al. Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science 280, 1262–1265 (1998).
Levin-Salomon, V., Kogan, K., Ahn, N.G., Livnah, O. & Engelberg, D. Isolation of intrinsically active (MEK-independent) variants of the ERK family of mitogen-activated protein (MAP) kinases. J. Biol. Chem. 283, 34500–34510 (2008).
Bogoyevitch, M.A. & Kobe, B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 70, 1061–1095 (2006).
Conde, C. & Caceres, A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 10, 319–332 (2009).
Poulain, F.E. & Sobel, A. The “SCG10-like protein” SCLIP is a novel regulator of axonal branching in hippocampal neurons, unlike SCG10. Mol. Cell. Neurosci. 34, 137–146 (2007).
Tararuk, T. et al. JNK1 phosphorylation of SCG10 determines microtubule dynamics and axodendritic length. J. Cell Biol. 173, 265–277 (2006).
Glorioso, C. et al. Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol. Psychiatry 11, 633–648 (2006).
Rico, B., Xu, B. & Reichardt, L.F. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat. Neurosci. 5, 225–233 (2002).
Miyoshi, G. et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J. Neurosci. 30, 1582–1594 (2010).
Balkowiec, A. & Katz, D.M. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J. Neurosci. 20, 7417–7423 (2000).
Brondello, J.M., Pouyssegur, J. & McKenzie, F.R. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science 286, 2514–2517 (1999).
Lin, Y.W. & Yang, J.L. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J. Biol. Chem. 281, 915–926 (2006).
Cox, L.J., Hengst, U., Gurskaya, N.G., Lukyanov, K.A. & Jaffrey, S.R. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat. Cell Biol. 10, 149–159 (2008).
Dorfman, K. et al. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene 13, 925–931 (1996).
Ibáñez, C.F. Message in a bottle: long-range retrograde signaling in the nervous system. Trends Cell Biol. 17, 519–528 (2007).
DiStefano, P.S. et al. The neurotrophins BDNF, NT-3 and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron 8, 983–993 (1992).
Baquet, Z.C., Gorski, J.A. & Jones, K.R. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J. Neurosci. 24, 4250–4258 (2004).
Waetzig, V. & Herdegen, T. Context-specific inhibition of JNKs: overcoming the dilemma of protection and damage. Trends Pharmacol. Sci. 26, 455–461 (2005).
Chang, L., Jones, Y., Ellisman, M.H., Goldstein, L.S. & Karin, M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev. Cell 4, 521–533 (2003).
Curmi, P.A. et al. Stathmin and its phosphoprotein family: general properties, biochemical and functional interaction with tubulin. Cell Struct. Funct. 24, 345–357 (1999).
Suh, L.H., Oster, S.F., Soehrman, S.S., Grenningloh, G. & Sretavan, D.W. L1/Laminin modulation of growth cone response to EphB triggers growth pauses and regulates the microtubule destabilizing protein SCG10. J. Neurosci. 24, 1976–1986 (2004).
Wise, S.P. & Jones, E.G. The organization and postnatal development of the commissural projection of the rat somatic sensory cortex. J. Comp. Neurol. 168, 313–343 (1976).
Bartkowska, K., Paquin, A., Gauthier, A.S., Kaplan, D.R. & Miller, F.D. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development 134, 4369–4380 (2007).
Alsina, B., Vu, T. & Cohen-Cory, S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat. Neurosci. 4, 1093–1101 (2001).
Markus, A., Patel, T.D. & Snider, W.D. Neurotrophic factors and axonal growth. Curr. Opin. Neurobiol. 12, 523–531 (2002).
Wu, J.J. et al. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab. 4, 61–73 (2006).
Jeanneteau, F., Garabedian, M.J. & Chao, M.V. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proc. Natl. Acad. Sci. USA 105, 4862–4867 (2008).
Saito, T. & Nakatsuji, N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev. Biol. 240, 237–246 (2001).
Meijering, E. et al. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 58, 167–176 (2004).
Acknowledgements
This research was supported by the National Alliance for Research on Schizophrenia and Depression (F.J. and G.M.), the Human Frontier Science Program Organization (K.D.) and grants from the US National Institutes of Health to M.V.C. (NS21072 and HD23315) and A.M.B. (R01 AR46504).
Author information
Authors and Affiliations
Contributions
F.J. and K.D. designed, performed and analyzed experiments. F.J., K.D. and M.V.C. wrote the manuscript. G.M. helped with the in utero electroporations. G.M. and A.M.B. provided mice and reagents.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 1368 kb)
Rights and permissions
About this article
Cite this article
Jeanneteau, F., Deinhardt, K., Miyoshi, G. et al. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat Neurosci 13, 1373–1379 (2010). https://doi.org/10.1038/nn.2655
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2655
This article is cited by
-
DUSP1/MKP-1 represents another piece in the P2X7R intracellular signaling puzzle in cerebellar cells: our last journey with Mª Teresa along the purinergic pathways of Eden
Purinergic Signalling (2023)
-
Role of dual specificity phosphatases (DUSPs) in melanoma cellular plasticity and drug resistance
Scientific Reports (2022)
-
PDGF-R inhibition induces glioblastoma cell differentiation via DUSP1/p38MAPK signalling
Oncogene (2022)
-
Rapid-acting antidepressants and the circadian clock
Neuropsychopharmacology (2022)
-
Valproic Acid: A Potential Therapeutic for Spinal Cord Injury
Cellular and Molecular Neurobiology (2021)