Abstract
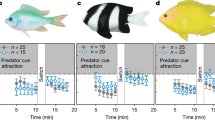
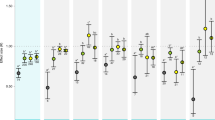
Predicted future CO2 levels have been found to alter sensory responses and behaviour of marine fishes. Changes include increased boldness and activity, loss of behavioural lateralization, altered auditory preferences and impaired olfactory function1,2,3,4,5. Impaired olfactory function makes larval fish attracted to odours they normally avoid, including ones from predators and unfavourable habitats1,3. These behavioural alterations have significant effects on mortality that may have far-reaching implications for population replenishment, community structure and ecosystem function2,6. However, the underlying mechanism linking high CO2 to these diverse responses has been unknown. Here we show that abnormal olfactory preferences and loss of behavioural lateralization exhibited by two species of larval coral reef fish exposed to high CO2 can be rapidly and effectively reversed by treatment with an antagonist of the GABA-A receptor. GABA-A is a major neurotransmitter receptor in the vertebrate brain. Thus, our results indicate that high CO2 interferes with neurotransmitter function, a hitherto unrecognized threat to marine populations and ecosystems. Given the ubiquity and conserved function of GABA-A receptors, we predict that rising CO2 levels could cause sensory and behavioural impairment in a wide range of marine species, especially those that tightly control their acid–base balance through regulatory changes in HCO3− and Cl− levels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Munday, P. L. et al. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl Acad. Sci. USA 106, 1848–1852 (2009).
Munday, P. L. et al. Replenishment of fish populations is threatened by ocean acidification. Proc. Natl Acad. Sci. USA 107, 12930–12934 (2010).
Dixson, D. L., Munday, P. L. & Jones, G. P. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 13, 68–75 (2010).
Simpson, S. D. et al. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 7, 917–920 (2011).
Domenici, P., Allan, B., McCormick, M. I. & Munday, P. L. Elevated CO2 affects behavioural lateralization in a coral reef fish. Biol. Lett. http://dx.doi.org/10.1098/rsbl.2011.0591 (2011).
Ferrari, M. C. O. et al. Putting prey and predator into the CO2 equation: Qualitative and quantitative effects of ocean acidification on predator–prey interactions. Ecol. Lett. 14, 1143–1148 (2011).
Meehl, G. A. et al. in IPCC Climate Change 2007: The Physical Science Basis (eds Solomon, S. et al.) 747–845 (Cambridge Univ. Press, 2007).
Doney, S. C., Fabry, V. J., Feely, R. A. & Kleypas, J. A. Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192 (2009).
Bormann, J., Hamill, O. P. & Sakmann, B. Mechanism of anion permeation through channels gated by glycine and γ-aminobutyric acid in mouse cultured spinal neurones. J. Physiol. 385, 243–286 (1987).
Lambert, N. & Grover, L. The mechanism of biphasic GABA responses. Science 269, 928–929 (1995).
Kahle, K. T. & Staley, K. Altered neuronal chloride homeostasis and excitatory GABAergic signaling in human temporal lobe epilepsy. Epilepsy Curr. 8, 51–53 (2008).
Kim, D. Y., Fenoglio, K. A., Kerrigan, J. F. & Rho, J. M. Bicarbonate contributes to GABA-A receptor mediated excitation in surgically resected human hypothalamic hamartomas. Epilepsy Res. 83, 89–93 (2009).
Ishimatsu, A., Hayashi, M. & Kikkawa, T. Fishes in high CO2 acidified oceans. Mar. Ecol. Prog. Ser. 373, 295–302 (2008).
Brauner, C. J. & Baker, D. W. in Cardio-Respiratory Control in Vertebrates (eds Glass, M. L. & Wood, S. C.) 43–63 (Springer, 2009).
Heaulme, M. et al. Biochemical characterization of the interaction of three pyridazinyl-GABA derivatives with the GABA A receptor site. Brain Res. 384, 224–231 (1986).
Fu, C., Wilson, J. M., Rombough, P. J. & Brauner, C. J. Ions first: Na+ uptake shifts from the skin to the gills before O2 uptake in developing rainbow trout, Oncorhynchus mykiss. Proc. R. Soc. B 277, 1553–1560 (2010).
Akerman, C. J. & Cline, H. T. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 30, 382–389 (2007).
Tsang, S. Y., Ng, S. K., Xu, Z. & Xue, H. The evolution of GABAA receptor-like genes. Mol. Biol. Evol. 24, 599–610 (2007).
Heisler, N. in Acid–Base Regulation in Animals (ed. Heisler, N.) 3–47 (Elsevier, 1986).
Pörtner, H. O., Langenbuch, M. & Reipschlager, A. Biological impact of elevated ocean CO2 concentrations: Lessons from animal physiology and earth history. J. Oceanogr. 60, 705–718 (2004).
Melzner, F. et al. Physiological basis for high CO2 tolerance in marine ectothermic animals: Pre-adaptation through lifestyle and ontogeny? Biogeoscience 6, 2313–2331 (2009).
Ohde, S. & Van Woesik, R. Carbon Dioxide flux and metabolic processes of a coral reef (Okinawa, Japan). Bull. Mar. Sci. 65, 559–576 (1999).
Kuffner, I. B., Andersson, A. J., Jokiel, P. L., Rodgers, K. S. & Mackenzie, F. T. Decreased abundance of crustose coralline algae due to ocean acidification. Nature Geosci. 1, 114–117 (2008).
Nilsson, G. E., Östlund-Nilsson, S., Penfold, R. & Grutter, A. S. From record performance to hypoxia tolerance—respiratory transition in damselfish larvae settling on a coral reef. Proc. R. Soc. B 274, 79–85 (2007).
Boutilier, R. G., Aughton, P. & Shelton, G. O2 and CO2 transport in relation to ventilation in Atlantic mackerel, Scomber scombrus. Can. J. Zool. 62, 546–554 (1984).
Gerlach, G., Atema, J., Kingsford, M. J., Black, K. P. & Miller-Sims, V. Smelling home can prevent dispersal of reef fish larvae. Proc. Natl Acad. Sci. USA 104, 858–863 (2007).
Thresher, R. E., Colin, P. L. & Bell, L. J. Planktonic duration, distribution and population structure of Western and Central Pacific Damselfishes (Pomacentridae). Copeia 1989, 420–434 (1989).
Meekan, M. G. A comparison of catches of fishes and invertebrates by two light trap designs, in tropical NW Australia. Mar. Biol. 139, 373–381 (2001).
Bisazza, A. et al. Lateralization of detour behaviour in poeciliid fish: The effect of species, gender and sexual motivation. Behav. Brain Res. 91, 157–164 (1998).
Dadda, M., Koolhaas, W. H. & Domenici, P. Behavioural asymmetry affects escape performance in a teleost fish. Biol. Lett. 6, 414–417 (2010).
Acknowledgements
We thank B. M. Devine, J. M. Donelson, G. M. Miller and the staff at Lizard Island Research Station for invaluable help with this study. The study was financially supported by The Australian Research Council and The University of Oslo.
Author information
Authors and Affiliations
Contributions
G.E.N. and P.L.M. devised the study. G.E.N., P.L.M. and P.D. designed the experiments. G.E.N., P.D., D.L.D., P.L.M., M.I.M. and C.S. conducted the experiments. S-A.W. developed equipment and conducted the chemical analyses. P.L.M. and P.D. conducted the statistical analyses. All authors contributed to writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Nilsson, G., Dixson, D., Domenici, P. et al. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nature Clim Change 2, 201–204 (2012). https://doi.org/10.1038/nclimate1352
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate1352
This article is cited by
-
Functional arrangement and temporal analyses of the coastal fish community of the southern Gulf of Mexico
Marine Biodiversity (2024)
-
Neuromolecular responses in disrupted mutualistic cleaning interactions under future environmental conditions
BMC Biology (2023)
-
Transgenerational exposure to ocean acidification impacts the hepatic transcriptome of European sea bass (Dicentrarchus labrax)
BMC Genomics (2023)
-
Short-term exposure to independent and combined acidification and warming elicits differential responses from two tropical seagrass-associated invertebrate grazers
Marine Biology (2023)
-
Effects of predation risk on the sensory asymmetries and defensive strategies of Bufotes balearicus tadpoles
Animal Cognition (2023)