Abstract
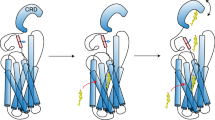
Oxysterols bind the seven-transmembrane protein Smo (Smo) and potently activate vertebrate Hedgehog (Hh) signaling, a pathway essential in embryonic development, adult stem cell maintenance and cancer. It is unknown, however, whether oxysterols are important for normal vertebrate Hh signaling and whether antagonizing oxysterols can inhibit the Hh pathway. We developed azasterols that block Hh signaling by binding the oxysterol-binding site of Smo. We show that the binding site for oxysterols and azasterols maps to the extracellular, cysteine-rich domain of Smo and is completely separable from the site bound by other small-molecule modulators, located within the heptahelical bundle of Smo. Smo mutants in which oxysterol binding is abolished no longer respond to oxysterols and cannot be maximally activated by the Hh ligand. Our results show that oxysterol binding to vertebrate Smo is required for normal Hh signaling and that targeting the oxysterol-binding site is an effective strategy to inhibit Smo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lum, L. & Beachy, P.A. The Hedgehog response network: sensors, switches, and routers. Science 304, 1755–1759 (2004).
Ingham, P.W. & McMahon, A.P. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087 (2001).
Taipale, J., Cooper, M.K., Maiti, T. & Beachy, P.A. Patched acts catalytically to suppress the activity of Smoothened. Nature 418, 892–897 (2002).
Chen, J.K., Taipale, J., Cooper, M.K. & Beachy, P.A. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 16, 2743–2748 (2002).
Frank-Kamenetsky, M. et al. Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J. Biol. 1, 10 (2002).
Robarge, K.D. et al. GDC-0449—a potent inhibitor of the hedgehog pathway. Bioorg. Med. Chem. Lett. 19, 5576–5581 (2009).
Chen, J.K., Taipale, J., Young, K.E., Maiti, T. & Beachy, P.A. Small molecule modulation of Smoothened activity. Proc. Natl. Acad. Sci. USA 99, 14071–14076 (2002).
Sinha, S. & Chen, J.K. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat. Chem. Biol. 2, 29–30 (2006).
Dwyer, J.R. et al. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J. Biol. Chem. 282, 8959–8968 (2007).
Corcoran, R.B. & Scott, M.P. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc. Natl. Acad. Sci. USA 103, 8408–8413 (2006).
Kim, W.K., Meliton, V., Amantea, C.M., Hahn, T.J. & Parhami, F. 20(S)-hydroxycholesterol inhibits PPARγ expression and adipogenic differentiation of bone marrow stromal cells through a hedgehog-dependent mechanism. J. Bone Miner. Res. 22, 1711–1719 (2007).
Nachtergaele, S. et al. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat. Chem. Biol. 8, 211–220 (2012).
Rohatgi, R., Milenkovic, L. & Scott, M.P. Patched1 regulates hedgehog signaling at the primary cilium. Science 317, 372–376 (2007).
Taipale, J. et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 406, 1005–1009 (2000).
Svärd, J. et al. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev. Cell 10, 187–197 (2006); erratum 10, 409.
Cooper, M.K. et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat. Genet. 33, 508–513 (2003).
Corbit, K.C. et al. Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018–1021 (2005).
Wang, Y., Zhou, Z., Walsh, C.T. & McMahon, A.P. Selective translocation of intracellular Smoothened to the primary cilium in response to Hedgehog pathway modulation. Proc. Natl. Acad. Sci. USA 106, 2623–2628 (2009).
Rohatgi, R., Milenkovic, L., Corcoran, R.B. & Scott, M.P. Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc. Natl. Acad. Sci. USA 106, 3196–3201 (2009).
Kim, J. et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell 17, 388–399 (2010).
Bazan, J.F. & de Sauvage, F.J. Structural ties between cholesterol transport and morphogen signaling. Cell 138, 1055–1056 (2009).
Wang, Y. et al. Glucocorticoid compounds modify smoothened localization and hedgehog pathway activity. Chem. Biol. 19, 972–982 (2012).
Janda, C.Y., Waghray, D., Levin, A.M., Thomas, C. & Garcia, K.C. Structural basis of Wnt recognition by Frizzled. Science 337, 59–64 (2012).
Dorn, K.V., Hughes, C.E. & Rohatgi, R.A. Smoothened–Evc2 complex transduces the Hedgehog signal at primary cilia. Dev. Cell 23, 823–835 (2012).
Infante, R.E. et al. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J. Biol. Chem. 283, 1064–1075 (2008).
Motamed, M. et al. Identification of luminal Loop 1 of Scap protein as the sterol sensor that maintains cholesterol homeostasis. J. Biol. Chem. 286, 18002–18012 (2011).
Nakano, Y. et al. Functional domains and sub-cellular distribution of the Hedgehog transducing protein Smoothened in Drosophila. Mech. Dev. 121, 507–518 (2004).
Aanstad, P. et al. The extracellular domain of Smoothened regulates ciliary localization and is required for high-level Hh signaling. Curr. Biol. 19, 1034–1039 (2009).
Tukachinsky, H., Lopez, L. & Salic, A. A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu–Gli protein complexes. J. Cell Biol. 191, 415–428 (2010).
Tukachinsky, H., Kuzmickas, R.P., Jao, C.Y., Liu, J. & Salic, A. Dispatched and scube mediate the efficient secretion of the cholesterol-modified hedgehog ligand. Cell Rep. 2, 308–320 (2012).
Klein, U., Gimpl, G. & Fahrenholz, F. Alteration of the myometrial plasma membrane cholesterol content with β-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry 34, 13784–13793 (1995).
Acknowledgements
We thank Y. Kishi and members of his laboratory for help with chiral chromatography and R. Rohatgi (Stanford University) for the initial gift of 20-OHC beads. A.S. is supported in part by US National Institutes of Health grant RO1 GM092924.
Author information
Authors and Affiliations
Contributions
D.N. and A.S. performed cellular and biochemical experiments. J.L., C.J. and A.S. designed and synthesized reported compounds. J.L. purified and characterized the compounds. Y.X. and D.N. developed automated image analysis software, and D.N. analyzed imaging data. All authors contributed data to the manuscript. A.S. wrote the manuscript, with input from all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–12, and Supplementary Note. (PDF 9872 kb)
Rights and permissions
About this article
Cite this article
Nedelcu, D., Liu, J., Xu, Y. et al. Oxysterol binding to the extracellular domain of Smoothened in Hedgehog signaling. Nat Chem Biol 9, 557–564 (2013). https://doi.org/10.1038/nchembio.1290
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1290
This article is cited by
-
Phosphorylation of human glioma-associated oncogene 1 on Ser937 regulates Sonic Hedgehog signaling in medulloblastoma
Nature Communications (2024)
-
De novo cholesterol biosynthesis in bacteria
Nature Communications (2023)
-
A proteome-wide map of 20(S)-hydroxycholesterol interactors in cell membranes
Nature Chemical Biology (2021)
-
ABT-199 inhibits Hedgehog pathway by acting as a competitive inhibitor of oxysterol, rather as a BH3 mimetic
Acta Pharmacologica Sinica (2021)
-
Cholesterol access in cellular membranes controls Hedgehog signaling
Nature Chemical Biology (2020)