Abstract
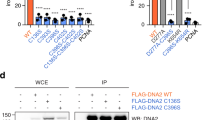
The eukaryotic replicative DNA polymerases (Pol α, δ and ɛ) and the major DNA mutagenesis enzyme Pol ζ contain two conserved cysteine-rich metal-binding motifs (CysA and CysB) in the C-terminal domain (CTD) of their catalytic subunits. Here we demonstrate by in vivo and in vitro approaches the presence of an essential [4Fe-4S] cluster in the CysB motif of all four yeast B-family DNA polymerases. Loss of the [4Fe-4S] cofactor by cysteine ligand mutagenesis in Pol3 destabilized the CTD and abrogated interaction with the Pol31 and Pol32 subunits. Reciprocally, overexpression of accessory subunits increased the amount of the CTD-bound Fe-S cluster. This implies an important physiological role of the Fe-S cluster in polymerase complex stabilization. Further, we demonstrate that the Zn-binding CysA motif is required for PCNA-mediated Pol δ processivity. Together, our findings show that the function of eukaryotic replicative DNA polymerases crucially depends on different metallocenters for accessory subunit recruitment and replisome stability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Johansson, E. & Macneill, S.A. The eukaryotic replicative DNA polymerases take shape. Trends Biochem. Sci. 35, 339–347 (2010).
Nick McElhinny, S.A., Gordenin, D.A., Stith, C.M., Burgers, P.M. & Kunkel, T.A. Division of labor at the eukaryotic replication fork. Mol. Cell 30, 137–144 (2008).
Pursell, Z.F., Isoz, I., Lundström, E.B., Johansson, E. & Kunkel, T.A. Yeast DNA polymerase ɛ participates in leading-strand DNA replication. Science 317, 127–130 (2007).
Burgers, P.M. et al. Eukaryotic DNA polymerases: proposal for a revised nomenclature. J. Biol. Chem. 276, 43487–43490 (2001).
Mäkiniemi, M. et al. A novel family of DNA-polymerase-associated B subunits. Trends Biochem. Sci. 24, 14–16 (1999).
Baranovskiy, A.G. et al. X-ray structure of the complex of regulatory subunits of human DNA polymerase δ. Cell Cycle 7, 3026–3036 (2008).
Nelson, J.R., Lawrence, C.W. & Hinkle, D.C. Thymine-thymine dimer bypass by yeast DNA polymerase ζ. Science 272, 1646–1649 (1996).
Prakash, S., Johnson, R.E. & Prakash, L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74, 317–353 (2005).
Bemark, M., Khamlichi, A.A., Davies, S.L. & Neuberger, M.S. Disruption of mouse polymerase ζ (Rev3) leads to embryonic lethality and impairs blastocyst development in vitro. Curr. Biol. 10, 1213–1216 (2000).
Krishna, S.S., Majumdar, I. & Grishin, N.V. Structural classification of zinc fingers: survey and summary. Nucleic Acids Res. 31, 532–550 (2003).
Tahirov, T.H., Makarova, K.S., Rogozin, I.B., Pavlov, Y.I. & Koonin, E.V. Evolution of DNA polymerases: an inactivated polymerase-exonuclease module in Pol ɛ and a chimeric origin of eukaryotic polymerases from two classes of archaeal ancestors. Biol. Direct 4, 11 (2009).
Swan, M.K., Johnson, R.E., Prakash, L., Prakash, S. & Aggarwal, A.K. Structural basis of high-fidelity DNA synthesis by yeast DNA polymerase δ. Nat. Struct. Mol. Biol. 16, 979–986 (2009).
Evanics, F., Maurmann, L., Yang, W.W. & Bose, R.N. Nuclear magnetic resonance structures of the zinc finger domain of human DNA polymerase-α. Biochim. Biophys. Acta 1651, 163–171 (2003).
Klinge, S., Núñez-Ramírez, R., Llorca, O. & Pellegrini, L. 3D architecture of DNA Pol α reveals the functional core of multi-subunit replicative polymerases. EMBO J. 28, 1978–1987 (2009).
Mizuno, T., Yamagishi, K., Miyazawa, H. & Hanaoka, F. Molecular architecture of the mouse DNA polymerase α-primase complex. Mol. Cell. Biol. 19, 7886–7896 (1999).
Dua, R., Levy, D.L. & Campbell, J.L. Analysis of the essential functions of the C-terminal protein/protein interaction domain of Saccharomyces cerevisiae pol ɛ and its unexpected ability to support growth in the absence of the DNA polymerase domain. J. Biol. Chem. 274, 22283–22288 (1999).
Sanchez Garcia, J., Ciufo, L.F., Yang, X., Kearsey, S.E. & MacNeill, S.A. The C-terminal zinc finger of the catalytic subunit of DNA polymerase δ is responsible for direct interaction with the B-subunit. Nucleic Acids Res. 32, 3005–3016 (2004).
Chanet, R. & Heude, M. Characterization of mutations that are synthetic lethal with pol3–13, a mutated allele of DNA polymerase delta in Saccharomyces cerevisiae. Curr. Genet. 43, 337–350 (2003).
Lill, R. Function and biogenesis iron-sulphur proteins. Nature 460, 831–838 (2009).
Hausmann, A. et al. The eukaryotic P-loop NTPase Nbp35: an essential component of the cytosolic and nuclear iron-sulfur protein assembly machinery. Proc. Natl. Acad. Sci. USA 102, 3266–3271 (2005).
Zhang, Y. et al. Dre2, a conserved eukaryotic Fe/S cluster protein, functions in cytosolic Fe/S protein biogenesis. Mol. Cell. Biol. 28, 5569–5582 (2008).
Netz, D.J.A. et al. Tah18 transfers electrons to Dre2 in cytosolic iron-sulfur protein biogenesis. Nat. Chem. Biol. 6, 758–765 (2010).
Roy, A., Solodovnikova, N., Nicholson, T., Antholine, W. & Walden, W.E. A novel eukaryotic factor for cytosolic Fe-S cluster assembly. EMBO J. 22, 4826–4835 (2003).
Netz, D.J.A., Pierik, A.J., Stümpfig, M., Mühlenhoff, U. & Lill, R. The Cfd1-Nbp35 complex acts as a scaffold for iron-sulfur protein assembly in the yeast cytosol. Nat. Chem. Biol. 3, 278–286 (2007).
Balk, J., Pierik, A.J., Netz, D.J.A., Mühlenhoff, U. & Lill, R. The hydrogenase-like Nar1p is essential for maturation of cytosolic and nuclear iron-sulphur proteins. EMBO J. 23, 2105–2115 (2004).
Balk, J., Netz, D.J.A., Tepper, K., Pierik, A.J. & Lill, R. The essential WD40 protein Cia1 is involved in a late step of cytosolic and nuclear iron-sulfur protein assembly. Mol. Cell. Biol. 25, 10833–10841 (2005).
Kispal, G., Csere, P., Prohl, C. & Lill, R. The mitochondrial proteins Atm1p and Nfs1p are required for biogenesis of cytosolic Fe/S proteins. EMBO J. 18, 3981–3989 (1999).
Klinge, S., Hirst, J., Maman, J.D., Krude, T. & Pellegrini, L. An iron-sulfur domain of the eukaryotic primase is essential for RNA primer synthesis. Nat. Struct. Mol. Biol. 14, 875–877 (2007).
Burgers, P.M. & Gerik, K.J. Structure and processivity of two forms of Saccharomyces cerevisiae DNA polymerase δ. J. Biol. Chem. 273, 19756–19762 (1998).
Orme-Johnson, W.H. & Orme-Johnson, A.R. Iron-sulfur proteins: the problem of determining cluster type. in Iron-Sulfur Proteins (ed. Spiro, T.G.) 67–95 (Wiley, 1982).
Kent, T.A. et al. Mössbauer studies of beef heart aconitase: evidence for facile interconversions of iron-sulfur clusters. Proc. Natl. Acad. Sci. USA 79, 1096–1100 (1982).
Tsurimoto, T. & Stillman, B. Multiple replication factors augment DNA synthesis by the two eukaryotic DNA polymerases, α and δ. EMBO J. 8, 3883–3889 (1989).
Johansson, E., Garg, P. & Burgers, P.M. The Pol32 subunit of DNA polymerase δ contains separable domains for processive replication and proliferating cell nuclear antigen (PCNA) binding. J. Biol. Chem. 279, 1907–1915 (2004).
Montecucco, A. et al. DNA ligase I is recruited to sites of DNA replication by an interaction with proliferating cell nuclear antigen: identification of a common targeting mechanism for the assembly of replication factories. EMBO J. 17, 3786–3795 (1998).
Hara, K. et al. Crystal structure of human REV7 in complex with a human REV3 fragment and structural implication of the interaction between DNA polymerase ζ and REV1. J. Biol. Chem. 285, 12299–12307 (2010).
Dauter, Z., Wilson, K.S., Sieker, L.C., Moulis, J.M. & Meyer, J. Zinc- and iron-rubredoxins from Clostridium pasteurianum at atomic resolution: a high-precision model of a ZnS4 coordination unit in a protein. Proc. Natl. Acad. Sci. USA 93, 8836–8840 (1996).
Ramelot, T.A. et al. Solution NMR structure of the iron-sulfur cluster assembly protein U (IscU) with zinc bound at the active site. J. Mol. Biol. 344, 567–583 (2004).
Urzica, E., Pierik, A.J., Mühlenhoff, U. & Lill, R. Crucial role of conserved cysteine residues in the assembly of two iron-sulfur clusters on the CIA protein Nar1. Biochemistry 48, 4946–4958 (2009).
Nar, H. et al. Characterization and crystal structure of zinc azurin, a by-product of heterologous expression in Escherichia coli of Pseudomonas aeruginosa copper azurin. Eur. J. Biochem. 205, 1123–1129 (1992).
Abbouni, B., Oehlmann, W., Stolle, P., Pierik, A.J. & Auling, G. Electron paramagnetic resonance (EPR) spectroscopy of the stable-free radical in the native metallo-cofactor of the manganese-ribonucleotide reductase (Mn-RNR) of Corynebacterium glutamicum. Free Radic. Res. 43, 943–950 (2009).
Boal, A.K., Cotruvo, J.A. Jr., Stubbe, J. & Rosenzweig, A.C. Structural basis for activation of class Ib ribonucleotide reductase. Science 329, 1526–1530 (2010).
Burgers, P.M.J. Saccharomyces cerevisiae replication factor C. II. Formation and activity of complexes with the proliferating cell nuclear antigen and with DNA polymerases δ and ɛ. J. Biol. Chem. 266, 22698–22706 (1991).
Garg, P., Stith, C.M., Majka, J. & Burgers, P.M.J. Proliferating cell nuclear antigen promotes translesion synthesis by DNA polymerase ζ. J. Biol. Chem. 280, 23446–23450 (2005).
Wong, S.W. et al. DNA polymerases α and δ are immunologically and structurally distinct. J. Biol. Chem. 264, 5924–5928 (1989).
Veatch, J.R., McMurray, M.A., Nelson, Z.W. & Gottschling, D.E. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell 137, 1247–1258 (2009).
Fortune, J.M., Stith, C.M., Kissling, G.E., Burgers, P.M. & Kunkel, T.A. RPA and PCNA suppress formation of large deletion errors by yeast DNA polymerase δ. Nucleic Acids Res. 34, 4335–4341 (2006).
Ayyagari, R., Impellizzeri, K.J., Yoder, B.L., Gary, S.L. & Burgers, P.M. A mutational analysis of the yeast proliferating cell nuclear antigen indicates distinct roles in DNA replication and DNA repair. Mol. Cell. Biol. 15, 4420–4429 (1995).
Gomes, X.V., Gary, S.L. & Burgers, P.M. Overproduction in Escherichia coli and characterization of yeast replication factor C lacking the ligase homology domain. J. Biol. Chem. 275, 14541–14549 (2000).
Henricksen, L.A., Umbricht, C.B. & Wold, M.S. Recombinant replication protein A: expression, complex formation, and functional characterization. J. Biol. Chem. 269, 11121–11132 (1994).
Acknowledgements
We thank B. Yoder for yeast two-hybrid analysis and M. Reuter for help with cloning. This work was supported by grant GM032431 from the US National Institutes of Health (P.M.J.B.), the Deutsche Forschungsgemeinschaft (SFB 593, Gottfried-Wilhelm Leibniz program, and GRK 1216), Rhön Klinikum, von Behring-Röntgen Stiftung, LOEWE-Program of State Hessen, Max-Planck Gesellschaft and Fonds der Chemischen Industrie.
Author information
Authors and Affiliations
Contributions
All authors performed experiments. D.J.A.N., R.L., P.M.J.B. and A.J.P. designed experiments, analyzed data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 1024 kb)
Rights and permissions
About this article
Cite this article
Netz, D., Stith, C., Stümpfig, M. et al. Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat Chem Biol 8, 125–132 (2012). https://doi.org/10.1038/nchembio.721
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.721
This article is cited by
-
What exactly does the PfK13 C580Y mutation in Plasmodium falciparum influence?
Parasites & Vectors (2023)
-
Heart Failure-Related Iron Deficiency Anemia Pathophysiology and Laboratory Diagnosis
Current Heart Failure Reports (2023)
-
Early adaptive responses in the skeletal muscle of young mice with hereditary hemochromatosis
Molecular Biology Reports (2023)
-
Manganese transporters regulate the resumption of replication in hydrogen peroxide-stressed Escherichia coli
BioMetals (2023)
-
The iron-sulfur cluster is essential for DNA binding by human DNA polymerase ε
Scientific Reports (2022)