Abstract
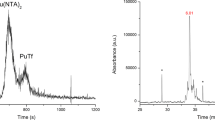
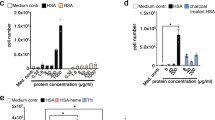
Plutonium is a toxic synthetic element with no natural biological function, but it is strongly retained by humans when ingested. Using small-angle X-ray scattering, receptor binding assays and synchrotron X-ray fluorescence microscopy, we find that rat adrenal gland (PC12) cells can acquire plutonium in vitro through the major iron acquisition pathway—receptor-mediated endocytosis of the iron transport protein serum transferrin; however, only one form of the plutonium–transferrin complex is active. Low-resolution solution models of plutonium-loaded transferrins derived from small-angle scattering show that only transferrin with plutonium bound in the protein's C-terminal lobe (C-lobe) and iron bound in the N-terminal lobe (N-lobe) (PuCFeNTf) adopts the proper conformation for recognition by the transferrin receptor protein. Although the metal-binding site in each lobe contains the same donors in the same configuration and both lobes are similar, the differences between transferrin's two lobes act to restrict, but not eliminate, cellular Pu uptake.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gorden, A.E.V., Xu, J., Raymond, K.N. & Durbin, P. Rational design of sequestering agents for plutonium and other actinides. Chem. Rev. 103, 4207–4282 (2003).
Durbin, P.W. Actinides in animals and man. in The Chemistry of the Actinide and Transactinide Elements Vol. 5 (eds. Morss, L.R., Edelstein, N.M. & Fuger, J.) 3339–3440 (Springer, Dordrecht, 2006).
Boocock, G. & Popplewell, D.S. Distribution of plutonium in serum proteins following intravenous injection into rats. Nature 208, 282–283 (1965).
Boocock, G., Danpure, C.J., Popplewell, D.S. & Taylor, D.M. The subcellular distribution of plutonium in rat liver. Radiat. Res. 42, 381–396 (1970).
Duffield, J.R. & Taylor, D.M. The biochemistry of the actinides. in Handbook on the Physics and Chemistry of the Actinides Vol. 4 (eds. Freeman, A.J. & Keller, C.) 129–157 (Elsevier Science, New York, 1986).
Taylor, D.M. Chemical and physical properties of plutonium. in Uranium, Plutonium, Transplutonic Elements Vol. 36, Handbook of Experimental Pharmacology (eds. Hodge, H.C., Stannard, J.N. & Hursh, J.) 323–347 (Springer-Verlag, New York, 1973).
Choppin, G.R. Solution chemistry of the actinides. Radiochim. Acta 32, 43–53 (1983).
Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Clark, D.L., Hecker, S.S., Jarvinen, G.D. & Neu, M.P. Plutonium. in The Chemistry of the Actinide and Transactinide Elements Vol. 2 (eds. Morss, L.R., Edelstein, N.M. & Fuger, J.) 813–1264 (Springer, Dordrecht, 2006).
Allard, B., Kipatsi, H. & Liljenzin, J.O. Expected species of uranium, neptunium and plutonium in neutral aqueous solutions. J. Inorg. Nucl. Chem. 42, 1015–1027 (1980).
Raymond, K.N. & Smith, W.L. Actinide-specific sequestering agents and decontamination applications. Struct. Bonding 43, 159–186 (1981).
Jeanson, A. et al. The role of transferrin in actinide(IV) uptake: comparison with iron(III). Chemistry 16, 1378–1387 (2010).
Taylor, D.M. The bioinorganic chemistry of actinides in blood. J. Alloys. Compd. 271–273, 6–10 (1998).
Sun, H., Li, H. & Sadler, P.J. Transferrin as a metal ion mediator. Chem. Rev. 99, 2817–2842 (1999).
Mason, A.B. et al. Mutational analysis of C-lobe ligands of human serum transferrin: insights into the mechanism of iron release. Biochemistry 44, 8013–8021 (2005).
Cheng, Y., Zak, O., Aisen, P., Harrison, S.C. & Walz, T. Structure of the human transferrin receptor-transferrin complex. Cell 116, 565–576 (2004).
Hoyes, K.P. et al. Transferrin-mediated uptake of plutonium by spermatogenic tubules. Int. J. Radiat. Biol. 70, 467–471 (1996).
Planas-Bohne, F. & Rau, W. Comparison of the binding of 59Fe- and 239Pu-transferrin to rat liver cell membranes. Hum. Exp. Toxicol. 9, 17–24 (1990).
Grossmann, J.G. et al. Metal-induced conformational changes in transferrins. J. Mol. Biol. 229, 585–590 (1993).
Duffield, J.R., Taylor, D.M. & Williams, D.R. The biochemistry of the f-elements. in Handbook on the Physics and Chemistry of Rare Earths Vol. 18 (eds. Gschneidner, K.A. Jr., Eyring, L., Choppin, G.R. & Lander, G.H.) 591–621 (Elsevier Science, New York, 1994).
Baker, E.N. Structure and reactivity of transferrins. in Advances in Inorganic Chemistry Vol. 41 (ed. Sykes, A.G.) 389–463 (Academic Press, San Diego, 1994).
Shih, Y.J. et al. Serum transferrin receptor is a truncated form of tissue receptor. J. Biol. Chem. 265, 19077–19081 (1990).
Giannetti, A.M., Snow, P.M., Zak, O. & Bjorkman, P.J. Mechanism for multiple ligand recognition by the human transferrin receptor. PLoS Biol. 1, E51 (2003).
Lebrón, J.A. et al. Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell 93, 111–123 (1998).
Grossmann, J.G. et al. X-ray solution scattering reveals conformational changes upon iron uptake in lactoferrin, serum and ovo-transferrins. J. Mol. Biol. 225, 811–819 (1992).
Svergun, D.I. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys. J. 76, 2879–2886 (1999).
Zuccola, H.J. The Crystal Structure of Monoferric Human Serum Transferrin. PhD thesis, Georgia Institute of Technology (1992).
Hall, D.R. et al. The crystal and molecular structures of diferric porcine and rabbit serum transferrin at resolutions of 2.15 and 2.60 Å, respectively. Acta Crystallogr. D Biol. Crystallogr. 58, 70–80 (2002).
Jeffrey, P.D. et al. Ligand-induced conformational change in transferrins: crystal structure of the open form of the N-terminal half-molecule of human transferrin. Biochemistry 37, 13978–13986 (1998).
Iacopetta, B.J. & Morgan, E.H. The kinetics of transferrin endocytosis and iron uptake from transferrin in rabbit reticulocytes. J. Biol. Chem. 258, 9108–9115 (1983).
Wessling-Resnick, M. Iron transport. Annu. Rev. Nutr. 20, 129–151 (2000).
Hagler, H.K., Kumar, V. & Robbins, S.L. Interactive Case Study Companion to Robbins Pathologic Basis of Disease 6th edn. (W.B. Saunders Co., 1999).
Leibman, A. & Aisen, P. Distribution of iron between the binding sites of transferrin in serum: methods and results in normal human subjects. Blood 53, 1058–1065 (1979).
Harris, W.R., Carrano, C.J., Pecoraro, V.L. & Raymond, K.N. Siderophilin metal coordination. 1. Complexation of thorium by transferrin: structure-function implications. J. Am. Chem. Soc. 103, 2231–2237 (1981).
Baker, H.M., Baker, C.J., Smith, C.A. & Baker, E.N. Metal substitution in transferrins: specific binding of cerium(IV) revealed by the crystal structure of cerium-substituted human lactoferrin. J. Biol. Inorg. Chem. 5, 692–698 (2000).
Park, I. et al. Organization of the human transferrin gene: direct evidence that it originated by gene duplication. Proc. Natl. Acad. Sci. USA 82, 3149–3153 (1985).
He, Q.Y. & Mason, A.B. Molecular aspects of release of iron from transferrins. in Molecular and Cellular Iron Transport (ed. Templeton, D.M.) 95–123 (Marcel Dekker, New York, 2002).
Duffield, J.R., Taylor, D.M. & Proctor, S.A. The binding of plutonium to transferrin in the presence of tri-n-butyl phosphate or nitrate and its release by diethylenetriaminepenta-acetate and the tetrameric catechoylamide ligand LICAMC(C). Int. J. Nucl. Med. Biol. 12, 483–487 (1986).
Durbin, P.W., Kullgren, B., Xu, J. & Raymond, K.N. Development of decorporation agents for the actinides. Radiat. Prot. Dosimetry 79, 433–443 (1998).
Bali, P.K. & Harris, W.R. Site-specific rate constants for iron removal from diferric transferrin by nitrilotris(methylenephosphonic acid) and pyrophosphate. Arch. Biochem. Biophys. 281, 251–256 (1990).
Chasteen, N.D. & Williams, J. The influence of pH on the equilibrium distribution of iron between the metal-binding sites of human transferrin. Biochem. J. 193, 717–727 (1981).
Hémadi, M., Miquel, G., Kahn, P.H. & El Hage Chahine, J.-M. Aluminum exchange between citrate and human serum transferrin and interaction with transferrin receptor 1. Biochemistry 42, 3120–3130 (2003).
Seifert, S., Winans, R.E., Tiede, D.M. & Thiyagarajan, P. Design and performance of a ASAXS instrument at the Advanced Photon Source. J. Appl. Crystallogr. 33, 782–784 (2000).
Fang, X. et al. Mg2+-dependent compaction and folding of yeast tRNAPhe and the catalytic domain of the B. subtilis RNase P RNA determined by small-angle X-ray scattering. Biochemistry 39, 11107–11113 (2000).
Antonio, M.R., Chiang, M.-H., Seifert, S., Tiede, D.M. & Thiyagarajan, P. In situ measurement of the Preyssler polyoxometallate morphology upon electrochemical reduction. J. Electroanal. Chem. 626, 103–110 (2009).
Konarev, P.V., Petoukhov, M.V., Volkov, V.V. & Svergun, D.I. Atsas 2.1, a program package for small-angle scattering data analysis. J. Appl. Crystallogr. 39, 277–286 (2006).
Cai, Z., Lai, B., Xiao, Y. & Xu, S. An X-ray diffraction microscope at the Advanced Photon Source. J. Phys. IV 104, 17–20 (2003).
McRae, R., Lai, B., Vogt, S. & Fahrni, C.J. Correlative microXRF and optical immunofluorescence microscopy of adherent cells labeled with ultrasmall gold particles. J. Struct. Biol. 155, 22–29 (2006).
Vogt, S. Maps: a set of software tools for analysis and visualization of 3D X-ray fluorescence data sets. J. Phys. IV France 104, 635–638 (2003).
Acknowledgements
The work at Argonne National Laboratory, including the use of the Advanced Photon Source, was supported by the US Department of Energy, Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357, whereas work at Northwestern University was supported by US National Institutes of Health grants EB002100 and U54CA119341.
Author information
Authors and Affiliations
Contributions
M.P.J., D.G.-L. and B.A. carried out the experiments and analyzed the data. T.P., P.G.R. and G.E.W. participated in the cell uptake experiments. T.P., L.S., G.E.W., S.V. and B.L. took part in the SXFM experiments and S.S. assisted with the SAXS measurements. S.S. and S.V. also worked on data reduction and analysis. M.P.J. designed the experiments. M.P.J., D.G.-L., B.A. and L.S. wrote the manuscript with input from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 3093 kb)
Rights and permissions
About this article
Cite this article
Jensen, M., Gorman-Lewis, D., Aryal, B. et al. An iron-dependent and transferrin-mediated cellular uptake pathway for plutonium. Nat Chem Biol 7, 560–565 (2011). https://doi.org/10.1038/nchembio.594
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.594
This article is cited by
-
Plutonium distribution in sequentially extracted phases of arable and uncultivated soils
Environmental Earth Sciences (2022)
-
Molecular dynamics simulations of plutonium binding and its decorporation from the binding-cleft of human serum transferrin
JBIC Journal of Biological Inorganic Chemistry (2020)
-
Fetuin exhibits a strong affinity for plutonium and may facilitate its accumulation in the skeleton
Scientific Reports (2019)
-
Iron and bismuth bound human serum transferrin reveals a partially-opened conformation in the N-lobe
Scientific Reports (2012)
-
Plutonium's Trojan horse
Nature Chemical Biology (2011)