Abstract
The sodium/iodide symporter (NIS) is the essential plasma membrane protein that mediates active iodide (I−) transport into the thyroid gland, the first step in the biosynthesis of the thyroid hormones—the master regulators of intermediary metabolism. NIS couples the inward translocation of I− against its electrochemical gradient to the inward transport of Na+ down its electrochemical gradient1,2. For nearly 50 years before its molecular identification3, NIS was the molecule at the centre of the single most effective internal radiation cancer therapy: radioiodide (131I−) treatment for thyroid cancer2. Mutations in NIS cause congenital hypothyroidism, which must be treated immediately after birth to prevent stunted growth and cognitive deficiency2. Here we report three structures of rat NIS, determined by single-particle cryo-electron microscopy: one with no substrates bound; one with two Na+ and one I− bound; and one with one Na+ and the oxyanion perrhenate bound. Structural analyses, functional characterization and computational studies show the substrate-binding sites and key residues for transport activity. Our results yield insights into how NIS selects, couples and translocates anions—thereby establishing a framework for understanding NIS function—and how it transports different substrates with different stoichiometries and releases substrates from its substrate-binding cavity into the cytosol.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Cryo-EM maps and atomic coordinates of the structures presented in this manuscript have been deposited in the Protein Data Bank (PDB) and Electron Microscopy Data Bank (EMDB) under accession codes 7UUY and EMD-26806 (for Apo-NIS), 7UV0 and EMD-26808 (for NIS-I−) and 7UUZ and EMD-26807 (for NIS-ReO4−). The trajectories and the Jupyter notebook used for molecular dynamics simulations are available at https://codeocean.com/capsule/1458259/tree.
References
Portulano, C., Paroder-Belenitsky, M. & Carrasco, N. The Na+/I− symporter (NIS): mechanism and medical impact. Endocr. Rev. 35, 106–149 (2014).
Ravera, S., Reyna-Neyra, A., Ferrandino, G., Amzel, L. M. & Carrasco, N. The sodium/iodide symporter (NIS): molecular physiology and preclinical and clinical applications. Annu. Rev. Physiol. 79, 261–289 (2017).
Dai, G., Levy, O. & Carrasco, N. Cloning and characterization of the thyroid iodide transporter. Nature 379, 458–460 (1996).
Mullur, R., Liu, Y. Y. & Brent, G. A. Thyroid hormone regulation of metabolism. Physiol. Rev. 94, 355–382 (2014).
Zhang, Z., Liu, F. & Chen, J. Molecular structure of the ATP-bound, phosphorylated human CFTR. Proc. Natl Acad. Sci. USA 115, 12757–12762 (2018).
Reyna-Neyra, A. et al. The iodide transport defect-causing Y348D mutation in the Na(+)/I(-) symporter renders the protein intrinsically inactive and impairs its targeting to the plasma membrane. Thyroid 31, 1272–1281 (2021).
Tazebay, U. H. et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat. Med. 6, 871–878 (2000).
Spitzweg, C. et al. The sodium iodide symporter (NIS): novel applications for radionuclide imaging and treatment. Endocr. Relat. Cancer 28, T193–T213 (2021).
Kitzberger, C. et al. The sodium iodide symporter (NIS) as theranostic gene: its emerging role in new imaging modalities and non-viral gene therapy. EJNMMI Res. 12, 25 (2022).
Urnauer, S. et al. EGFR-targeted nonviral NIS gene transfer for bioimaging and therapy of disseminated colon cancer metastases. Oncotarget 8, 92195–92208 (2017).
Miller, A. & Russell, S. J. The use of the NIS reporter gene for optimizing oncolytic virotherapy. Expert Opin. Biol. Ther. 16, 15–32 (2016).
Li, W., Nicola, J. P., Amzel, L. M. & Carrasco, N. Asn441 plays a key role in folding and function of the Na+/I− symporter (NIS). FASEB J. 27, 3229–3238 (2013).
Nicola, J. P. et al. Sodium/iodide symporter mutant V270E causes stunted growth but no cognitive deficiency. J. Clin. Endocrinol. Metab. 100, E1353–E1361 (2015).
Paroder-Belenitsky, M. et al. Mechanism of anion selectivity and stoichiometry of the Na+/I- symporter (NIS). Proc. Natl Acad. Sci. USA 108, 17933–17938 (2011).
Levy, O. et al. N-linked glycosylation of the thyroid Na+/I− symporter (NIS). Implications for its secondary structure model. J. Biol. Chem. 273, 22657–22663 (1998).
Levy, O. et al. Characterization of the thyroid Na+/I− symporter with an anti-COOH terminus antibody. Proc. Natl Acad. Sci. USA 94, 5568–5573 (1997).
Paroder, V., Nicola, J. P., Ginter, C. S. & Carrasco, N. The iodide transport defect-causing mutation R124H: a δ-amino group at position 124 is critical for maturation and trafficking of the Na+/I- symporter (NIS). J. Cell Sci. 126, 3305–3313 (2013).
De la Vieja, A., Reed, M. D., Ginter, C. S. & Carrasco, N. Amino acid residues in transmembrane segment IX of the Na+/I− symporter play a role in its Na+ dependence and are critical for transport activity. J. Biol. Chem. 282, 25290–25298 (2007).
Chew, T. A. et al. Structure and mechanism of the cation-chloride cotransporter NKCC1. Nature 572, 488–492 (2019).
Coleman, J. A., Green, E. M. & Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 532, 334–339 (2016).
Han, L. et al. Structure and mechanism of the SGLT family of glucose transporters. Nature 601, 274–279(2022).
Niu, Y. et al. Structural basis of inhibition of the human SGLT2-MAP17 glucose transporter. Nature 601, 280–284 (2021).
Yamashita, A., Singh, S. K., Kawate, T., Jin, Y. & Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl-dependent neurotransmitter transporters. Nature 437, 215–223 (2005).
Faham, S. et al. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science 321, 810–814 (2008).
Wang, J., Liu, Z., Frank, J. & Moore, P. B. Identification of ions in experimental electrostatic potential maps. IUCrJ 5, 375–381 (2018).
Zhekova, H. R. et al. Mapping of ion and substrate binding sites in human sodium iodide symporter (hNIS). J. Chem. Inf. Model. 60, 1652–1665 (2020).
Nicola, J. P., Carrasco, N. & Amzel, L. M. Physiological sodium concentrations enhance the iodide affinity of the Na+/I− symporter. Nat. Commun. 5, 3948 (2014).
Ravera, S., Quick, M., Nicola, J. P., Carrasco, N. & Amzel, L. M. Beyond non-integer Hill coefficients: a novel approach to analyzing binding data, applied to Na+-driven transporters. J. Gen. Physiol. 145, 555–563 (2015).
Dohan, O. et al. The Na+/I symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate. Proc. Natl Acad. Sci. USA 104, 20250–20255 (2007).
Tran, N. et al. Thyroid-stimulating hormone increases active transport of perchlorate into thyroid cells. Am. J. Physiol. Endocrinol. Metab. 294, E802–E806 (2008).
Zuckier, L. S. et al. Kinetics of perrhenate uptake and comparative biodistribution of perrhenate, pertechnetate, and iodide by NaI symporter-expressing tissues in vivo. J. Nucl. Med. 45, 500–507 (2004).
Eskandari, S. et al. Thyroid Na+/I- symporter. Mechanism, stoichiometry, and specificity. J. Biol. Chem. 272, 27230–27238 (1997).
Llorente-Esteban, A. et al. Allosteric regulation of mammalian Na+/I− symporter activity by perchlorate. Nat. Struct. Mol. Biol. 27, 533–539 (2020).
Boutagy, N. E. et al. Noninvasive in vivo quantification of adeno-associated virus serotype 9-mediated expression of the sodium/iodide symporter under hindlimb ischemia and neuraminidase desialylation in skeletal muscle using single-photon emission computed tomography/computed tomography. Circ. Cardiovasc. Imaging 12, e009063 (2019).
Pavelka, A. et al. CAVER: algorithms for analyzing dynamics of tunnels in macromolecules. IEEE/ACM Trans. Comput. Biol. Bioinform. 13, 505–517 (2016).
Ferrandino, G. et al. Na+ coordination at the Na2 site of the Na+/I− symporter. Proc. Natl Acad. Sci. USA 113, E5379–E5388 (2016).
Sun, L. et al. Molecular dynamics simulations of the surface tension and structure of salt solutions and clusters. J. Phys. Chem. 116, 3198–3204 (2012).
Carugo, O. Buried chloride stereochemistry in the Protein Data Bank. BMC Struct. Biol. 14, 19 (2014).
Kang, B., Tang, H., Zhao, Z. & Song, S. Hofmeister series: insights of ion specificity from amphiphilic assembly and interface property. ACS Omega 5, 6229–6239 (2020).
Marcus, Y. A simple empirical model describing the thermodynamics of hydration of ions of widely varying charges, sizes, and shapes. Biophys. Chem. 51, 111–127 (1994).
Yang, D. & Gouaux, E. Illumination of serotonin transporter mechanism and role of the allosteric site. Sci. Adv. 7, eabl3857 (2021).
Coleman, J. A. & Gouaux, E. Structural basis for recognition of diverse antidepressants by the human serotonin transporter. Nat. Struct. Mol. Biol. 25, 170–175 (2018).
Levy, O., Ginter, C. S., De la Vieja, A., Levy, D. & Carrasco, N. Identification of a structural requirement for thyroid Na+/I- symporter (NIS) function from analysis of a mutation that causes human congenital hypothyroidism. FEBS Lett. 429, 36–40 (1998).
Ferrandino, G. et al. Na+ coordination at the Na2 site of the Na+/I- symporter. Proc. Natl Acad. Sci. USA 113, E5379–E5388 (2016).
Ravera, S., Quick, M., Nicola, J. P., Carrasco, N. & Amzel, L. M. Beyond non-integer Hill coefficients: a novel approach to analyzing binding data, applied to Na+-driven transporters. J. Gen. Physiol. 145, 555–563 (2015).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Moriya, T. et al. Size matters: optimal mask diameter and box size for single-particle cryogenic electron microscopy. Preprint at bioRxiv https://doi.org/10.1101/2020.08.23.263707 (2020).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Górski, K. M. et al. HEALPix: a framework for high-resolution discretization and fast analysis of data distributed on the sphere. Astrophys. J. 622, 759–771 (2005).
Wang, J., Liu, Z., Frank, J. & Moore, P. B. Identification of ions in experimental electrostatic potential maps. IUCrJ 5, 375–381 (2018).
Lee, J. et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 12, 405–413 (2016).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1, 19–25 (2015).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Hess, B., Bekker, H., Berendsen, H. J. & Fraaije, J. G. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Li, P., Song, L. F. & Merz, K. M. Jr. Systematic parameterization of monovalent ions employing the nonbonded model. J. Chem. Theory Comput. 11, 1645–1657 (2015).
Acknowledgements
We thank H. Mchaourab and members of the Carrasco laboratory for critical reading of the manuscript and insightful discussion, and S. Gabelli for helpful suggestions. This study was supported by National Institutes of Health (NIH) grants GM R01 114250 (to N.C. and L.M.A.), NS021501 (to F.J.S.) and NINDS R21NS108842, and a Pamela Mars Wright Innovator Award (to M.A.B.). We thank S. Wu of the Yale Cryo-EM Resource facility for screening and data collection. This work was conducted in part using the CPU and GPU resources at the Advanced Computing Center for Research and Education at Vanderbilt University and at the Maryland Advanced Research Computing Center. We used the DORS storage system supported by NIH (no. S10RR031634 to J. Smith).
Author information
Authors and Affiliations
Contributions
S.R., N.C. and L.M.A. conceived the project. S.R. expressed and purified proteins and prepared cryo-grids. S.R. and E.K. processed cryo-EM data. S.R., E.K., L.M.A. and M.A.B. built and refined the atomic models. F.J.S. implemented the protocol for ion identification. S.R. and M.A.B. carried out molecular dynamics simulations. S.R., J.P.N. and G.S.S. generated mutant NIS proteins and carried out functional assays. S.R. and N.C. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Jue Chen, Wojciech Kopec, Christine Spitzweg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Cryo-EM data processing and determination of the structure of NIS.
Topology model of the engineered NIS molecule (T-NIS) whose cDNA was used to transduce 293F cells. We engineered an HA tag onto the N-terminus and a HIS and an SBP (streptavidin-binding protein) tag onto the C-terminus for affinity purification. The tags are separated from NIS by a TEV protease site at the N-terminus and a PreScission site at the C-terminus. b. Enrichment of 293F cells expressing NIS by flow cytometry using an anti-HA antibody. After two rounds of sorting, >96% of the cells expressed NIS at the plasma membrane. c. I− transport assay. T-NIS transports virtually as much I− as wild-type (WT) NIS does, whereas nontransduced (NT) cells transport no I− . Results are expressed as pmol of I− accumulated/µg DNA ± s.e.m. Values represent averages of the results from two different experiments, each of which was carried out in triplicate (n = 6). d. Size exclusion chromatography (SEC) of the fraction of NIS purified in LMNG/GDN used for cryo-EM imaging detected by Trp-fluorescence. e. Coomassie blue staining of SEC-purified NIS subjected to SDS-PAGE (representative gel (n=3); see also Supplementary Fig. 1). f. Cryo-EM micrograph of apo-NIS (representative of 8025 micrographs that yielded similar results). The scale bar represents 200 nm. g. Selected 2D class averages obtained after 4 rounds of 2D classification using Relion (top image) and data-processing workflow (bottom two rows). h. Fourier shell correlation (FSC) of the locally refined map. i. NIS dimer map colored according to local resolution. j. Fitting of NIS sequence to the electron density map. k. Local refinement.
Extended Data Fig. 2 Cryo-EM densities and model of TMSs and dimeric assembly of NIS.
a. α-helical features are clearly visible in all 13 TMSs. b. NIS structure viewed from the extracellular side of the membrane, with the numbered TMs depicted as cylinders (left panel). NIS embedded in the membrane, top view; an example of 2D classes representing the corresponding view is shown in the black square (middle panel). NIS structure viewed from the intracellular side of the membrane, with the numbered TMs depicted as cylinders (right panel). c. Residues interacting at the dimer interface in apo-NIS.
Extended Data Fig. 3 Cryo-EM data processing and determination of the structures of NIS with substrates bound.
a. NIS-I−. b. NIS-ReO4− .
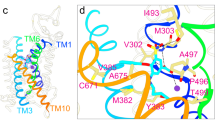
Extended Data Fig. 4 I− binds to a partially positively charged cavity.
a. NIS-I− side view. The solvent accessible surface is colored according to the electrostatic potential using a double gradient between −2 EV (red) and 0 (white) and between 0 and 2 EV (blue) and cropped to expose the I− binding cavity. b. Close-up of the I− binding cavity showing the positive nature of its surface.
Extended Data Fig. 5 Effects of single amino acid substitutions at positions 69, 72, 144, 416, 417 on iodide transport.
a–e. NIS-mediated I− uptake at steady state. cDNA constructs coding for NIS mutants were transfected into COS7 or HEK cells. I− uptake by these NIS mutants was measured at 20 µM (light gray bars) and 200 µM (dark gray bars) I− at 140 mM Na+ for 30 min with or without the NIS-specific inhibitor ClO4− to determine NIS-mediated transport (values obtained in the presence of ClO4−, which are < 10% of the values obtained in its absence, have already been subtracted). f–j. Kinetic analysis of initial rates of I− uptake (2-min time points) determined at 140 mM Na+ and varying concentrations of I−. k–o. Kinetic analysis of initial rates of I− uptake (2-min time points) determined at varying concentrations of extracellular Na+. All results are expressed as pmol of I− accumulated/µg DNA ± s.e.m. Values represent averages of the results from two or three different experiments, each of which was carried out in triplicate (n ≥ 6).
Extended data Fig. 6 Identification of the ions in the NIS-ReO4− structure.
a. The ions transported by NIS were identified by evaluating the map density along 24 lines passing through each site (circled in yellow on images of map slices). Values are plotted for each line and the spherically-averaged mean is plotted in black (lower panels). b. Effects of substitutions in binding-site residues on ReO4− transport at steady state (measured at 3 µM ReO4− and 140 mM Na+ for 30 min (values obtained in the presence of ClO4−, which are < 10% of the values obtained in its absence, have already been subtracted). Values are normalized to those obtained with WT NIS. Values represent averages of the results from two or three different experiments, each of which was carried out in triplicate (n ≥ 6) and reported as pmol ReO4− accumulated/µg DNA ± s.e.m.
Extended Data Fig. 7 Effects of single amino acid substitutions at positions 72, 94, 416 and 417 on ReO4− transport.
a-d. NIS-mediated ReO4− uptake at steady state. cDNA constructs coding for NIS mutants in which Q72 is replaced with the residues indicated were transfected into COS7 or HEK cells. ReO4− uptake by these NIS mutants was measured at 3 µM (light gray bars) and 30 µM (dark gray bars) ReO4− at 140 mM Na+ for 30 min with or without the NIS-specific inhibitor ClO4− (values obtained in the presence of ClO4− already subtracted). Results are given as pmols of ReO4− accumulated/µg DNA ± s.e.m. Values represent averages of the results from two or three different experiments, each of which was carried out in triplicate (n ≥ 6). e. Kinetic analysis of initial rates of ReO4− uptake (2-min time points) for Q72 NIS mutants determined at varying concentrations of extracellular ReO4− and varying concentrations of extracellular Na+. Results are given as pmols of ReO4− accumulated/µg DNA ± s.e.m. Values represent averages of the results from two or three different experiments, each of which was carried out in triplicate (n ≥ 6). f. ReO4− KM values determined from (e); error bars represent the standard deviation of the Michaelis-Menten analysis. g. Kinetic analysis of initial rates of ReO4− uptake (2-min time points) for Q72 NIS mutants determined at 100 µM ReO4− and varying concentrations of extracellular Na+. Results are given as pmols of ReO4− accumulated/µg DNA ± s.e.m. Values represent averages of the results from two or three different experiments, each of which was carried out in triplicate (n ≥ 6). h. Na+ KM values determined from (g); error bars represent the standard deviation of the Hill equation analysis.
Extended Data Fig. 8 Entry pathway for the NIS substrates.
a. Surface representation of a side view of the NIS-I− structure: the arrow and the dotted square indicate the position of the proposed entry pathway. b. Close-up of the top view of the surface showing the substrates (Na+ ions represented by yellow spheres, I− by a magenta sphere), and the positions of F87, L413, and Q414 in the NIS-I− structure (magenta) and the models generated from MD simulations corresponding to the opening (wheat) of the substrate-binding cavity to the extracellular milieu. c. Magnification of the top of the substrate-binding cavity. The arrows indicate how the amino acids move away from their original positions as the cavity transitions from closed to open. d. Ramachandran plots of the chi-1 and chi-2 side chain dihedral angles of F87, L413, and Q414 visited during the MD simulations with NIS-I−. The dihedral angles selected are the principal determinants of the position of the side chain. The excursions of these dihedral angles (during the MD simulations) away from the conformational basins corresponding to the cryoEM structure (green dots in basins 1, 1, and 2, in F87, L413, and Q414, respectively) and toward conformational basins (blue dots in basins 1, 2, and 3, in F87, L413, and Q414, respectively) open up the entry path (b). In these histograms, the frequency of a given conformational state is indicated by a rainbow gradient from deep purple (0 frequency) to red (highest frequency); the most highly populated conformational basins are numbered in descending order of population.
Extended Data Fig. 9 Perrhenate binding mechanism starting from the apo-NIS structure.
A hydrogen-bond network between F67, S69, Q72, and Y144 and a hydrophobic stacking interaction between F67 and Q72 are disrupted by the binding of the first Na+. This facilitates the binding of a second Na+ and ReO4−, which causes the release of one Na+.
Extended Data Fig. 10 Apo-NIS structure with amino acids mutated in patients with iodide transport defects shown as spheres.
Single amino acid substitutions are indicated by red spheres; deleted residues are in blue. The substitutions found in patients are: G18R, V59E, G93R, R124H, Q267E, V270E, D331N, Y348D, T354P, G395R, G543E, S547R, G561E and the deletion of 439-443 (ACNTP).
Supplementary information
Supplementary Information
Supplementary Figs. 1–11 and Table 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ravera, S., Nicola, J.P., Salazar-De Simone, G. et al. Structural insights into the mechanism of the sodium/iodide symporter. Nature 612, 795–801 (2022). https://doi.org/10.1038/s41586-022-05530-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05530-2
This article is cited by
-
Genomic alterations in thyroid cancer: biological and clinical insights
Nature Reviews Endocrinology (2024)
-
Ion and lipid orchestration of secondary active transport
Nature (2024)
-
Transport mechanism of presynaptic high-affinity choline uptake by CHT1
Nature Structural & Molecular Biology (2024)
-
Structures of human SGLT in the occluded state reveal conformational changes during sugar transport
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.