Abstract
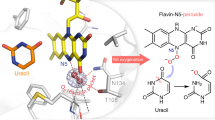
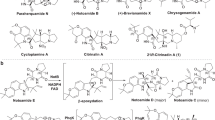
Despite the remarkable versatility displayed by flavin-dependent monooxygenases (FMOs) in natural product biosynthesis, one notably missing activity is the oxidative generation of carbonate functional groups. We describe a multifunctional Baeyer-Villiger monooxygenase, CcsB, which catalyzes the formation of an in-line carbonate in the macrocyclic portion of cytochalasin E. This study expands the repertoire of activities of FMOs and provides a possible synthetic strategy for transformation of ketones into carbonates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Huijbers, M.M., Montersino, S., Westphal, A.H., Tischler, D. & van Berkel, W.J. Arch. Biochem. Biophys. 544, 2–17 (2014).
Walsh, C.T. & Wencewicz, T.A. Nat. Prod. Rep. 30, 175–200 (2013).
Teufel, R. et al. Nature 503, 552–556 (2013).
Tsunematsu, Y. et al. Nat. Chem. Biol. 9, 818–825 (2013).
Francisco, W.A., AbuSoud, H.M., Topgi, R., Baldwin, T.O. & Raushel, F.M. J. Biol. Chem. 271, 104–110 (1996).
de Gonzalo, G., Mihovilovic, M.D. & Fraaije, M.W. ChemBioChem 11, 2208–2231 (2010).
Leisch, H., Morley, K. & Lau, P.C.K. Chem. Rev. 111, 4165–4222 (2011).
Zhang, H., Liu, H.B. & Yue, J.M. Chem. Rev. 114, 883–898 (2014).
Tomoda, H. et al. J Antibiot. (Tokyo) 52, 851–856 (1999).
Pongcharoen, W., Rukachaisirikul, V., Phongpaichit, S., Rungjindamai, N. & Sakayaroj, J. J. Nat. Prod. 69, 856–858 (2006).
Scherlach, K., Boettger, D., Remme, N. & Hertweck, C. Nat. Prod. Rep. 27, 869–886 (2010).
Vederas, J.C. J. Am. Chem. Soc. 102, 374–376 (1980).
Qiao, K., Chooi, Y.H. & Tang, Y. Metab. Eng. 13, 723–732 (2011).
Mirza, I.A. et al. J. Am. Chem. Soc. 131, 8848–8854 (2009).
Malito, E., Alfieri, A., Fraaije, M.W. & Mattevi, A. Proc. Natl. Acad. Sci. USA 101, 13157–13162 (2004).
Ishiuchi, K. et al. J. Am. Chem. Soc. 135, 7371–7377 (2013).
Kimura, Y., Nakajima, H. & Hamasaki, T. Agric. Biol. Chem. 53, 1699–1701 (1989).
Lin, Z.J. et al. Helv. Chim. Acta 92, 1538–1544 (2009).
Minato, H. & Matsumoto, M. J. Chem. Soc. Perkin 1 1, 38–45 (1970).
Rubin, M.B. & Inbar, S. J. Org. Chem. 53, 3355–3358 (1988).
Hong, X., Mejia-Oneto, J.M. & Padwa, A. Tetrahedr. Lett. 47, 8387–8390 (2006).
Mejía-Oneto, J.M. & Padwa, A. Helv. Chim. Acta 91, 285–302 (2008).
Robert, J.L. & Tamm, C. Helv. Chim. Acta 58, 2501–2504 (1975).
Frank, B. et al. J. Mol. Biol. 374, 24–38 (2007).
Tang, M.C., He, H.Y., Zhang, F. & Tang, G.L. ACS Catal. 3, 444–447 (2013).
Yelton, M.M., Hamer, J.E. & Timberlake, W.E. Proc. Natl. Acad. Sci. USA 81, 1470–1474 (1984).
Acknowledgements
This work was supported by the US National Institutes of Health (1R01GM085128 and 1DP1GM106413) to Y.T., the Natural Sciences and Engineering Research Council of Canada and the Canada Research Chair in Bioorganic and Medicinal Chemistry to J.C.V. and the US National Science Foundation (NSF) CHE-1059084 to K.N.H. A.P. thanks the Chemistry Biology Interface program (T32GM008496) for support. NMR instrumentation was supported by the NSF equipment grant CHE-1048804. We thank Y.-H. Chooi and N.K. Garg for helpful discussions. We thank S.I. Khan at the University of California–Los Angeles Department of Chemistry and Biochemistry crystallography facility for solving the X-ray structures. We thank D. Li at Chinese Ocean University for providing the standards for 5, 9 and 10. We acknowledge the Extreme Science and Engineering Design Environment program (TG-CHE040013N) for providing high-performance computing resources.
Author information
Authors and Affiliations
Contributions
Y.H., D.D., J.C.V. and Y.T. developed the hypothesis and designed the study. D.D. performed the isotopic labeling studies in A. clavatus, and J.A.J.T. and D.D. prepared selected substrate analogs. Y.H. performed the compound isolation and characterization. Y.H. performed the in vitro analysis of CcsB functions. A.P. and K.N.H. performed and interpreted the density functional theory calculations. All of the authors analyzed and discussed the results. Y.H., J.C.V. and Y.T. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–7, Supplementary Figures 1–33 and Supplementary Notes 1–3. (PDF 16669 kb)
Supplementary Data Set 1
CIF file for the crystal structure of cytochalasin Z16 (9), CCDC 970432 (TXT 591 kb)
Supplementary Data Set 2
CIF file for the crystal structure of ketocytochalasin (7), CCDC 970431 (TXT 933 kb)
Supplementary Data Set 3
Checkcif output file for CIF file of 9. (PDF 214 kb)
Supplementary Data Set 4
Checkcif output file for CIF file of 7. (PDF 231 kb)
Rights and permissions
About this article
Cite this article
Hu, Y., Dietrich, D., Xu, W. et al. A carbonate-forming Baeyer-Villiger monooxygenase. Nat Chem Biol 10, 552–554 (2014). https://doi.org/10.1038/nchembio.1527
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1527
This article is cited by
-
Enhanced production of aspochalasin D through genetic engineering of Aspergillus flavipes
Applied Microbiology and Biotechnology (2023)
-
Berberine bridge enzyme-like oxidase-catalysed double bond isomerization acts as the pathway switch in cytochalasin synthesis
Nature Communications (2022)
-
A new class of dimeric product isolated from the fungus Chaetomium globosum: evaluation of chemical structure and biological activity
The Journal of Antibiotics (2020)
-
Discovery and characterization of a cytochalasan biosynthetic cluster from the marine-derived fungus Aspergillus flavipes CNL-338
The Journal of Antibiotics (2020)
-
A pyridoxal phosphate–dependent enzyme that oxidizes an unactivated carbon-carbon bond
Nature Chemical Biology (2016)