Abstract
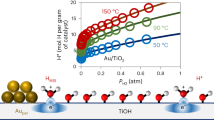
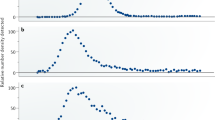
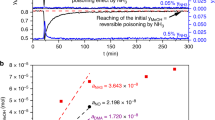
In heterogeneous catalysis, rates with Arrhenius-like temperature dependence are ubiquitous. Compensation phenomena, which arise from the linear correlation between the apparent activation energy and the logarithm of the apparent pre-exponential factor, are also common. Here, we study the origin of compensation and find a similar dependence on the rate-limiting surface coverage term for each Arrhenius parameter. This result is derived from an experimental determination of the surface coverage of oxygen and chlorine species using temporal analysis of products and prompt gamma activation analysis during HCl oxidation to Cl2 on a RuO2 catalyst. It is also substantiated by theory. We find that compensation phenomena appear when the effect on the apparent activation energy caused by changes in surface coverage is balanced out by the entropic configuration contributions of the surface. This result sets a new paradigm in understanding the interplay of compensation effects with the kinetics of heterogeneously catalysed processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ertl, G., Knötzinger, H., Schüth, F. & Weitkamp J. (eds) Handbook of Heterogeneous Catalysis (Wiley-VCH, 2008).
Constable, F. H. The mechanism of catalytic decomposition. Proc. R. Soc. Lond. A 108, 355–378 (1925).
Cremer, E. The compensation effect in heterogeneous catalysis. Adv. Catal. 7, 75–91 (1955).
Wilson, M. C. & Galwey, A. K. Compensation effect in heterogeneous catalytic reactions including hydrocarbon formation on clays. Nature 243, 402–404 (1973).
Bond, G. C., Keane, M. A., Kral, H. & Lercher, J. A. Compensation phenomena in heterogeneous catalysis: general principles and a possible explanation. Catal. Rev. Sci. Eng. 42, 323–383 (2000).
Hibi, T., Nishida, H. & Abekawa, H. Process for producing chlorine. US patent 5,871,707 (1999).
Hibi, T. et al. Process for producing chlorine. European patent EP936184 (1999).
Wolf, A., Mleczko, L., Schlüter, O. F. & Schubert, S. Method for producing chlorine by gas phase oxidation. European patent EP2026905 ( 2006).
Wolf, A., Mleczko, L., Schubert, S. & Schlüter, O. F. Method for producing chlorine by gas phase oxidation. European patent EP2027063 ( 2006).
Seki, K. Development of RuO2/rutile-TiO2 catalyst for industrial HCl oxidation process. Catal. Surv. Asia 14, 168–175 (2010).
Mondelli, C., Amrute, A. P., Schmidt, T. & Pérez-Ramírez, J. Shaped RuO2/SnO2-Al2O3 catalyst for large-scale stable Cl2 production by HCl oxidation. ChemCatChem. 3, 657–660 (2011).
Liu, L. & Guo, Q. X. Isokinetic relationship, isoequilibrium relationship, and enthalpy–entropy compensation. Chem. Rev. 101, 673–695 (2001).
Bligaard, T. et al. On the compensation effect in heterogeneous catalysis. J. Phys. Chem. B 107, 9325–9331 (2003).
Lynggaard, H., Andreasen, A., Stegelmann, C. & Soltze, P. Analysis of simple kinetic models in heterogeneous catalysis. Prog. Surf. Sci. 77, 71–137 (2004).
Kang, H. C., Jachimowski, T. A. & Weinberg, W. H. Role of local configurations in a Langmuir–Hinshelwood surface reaction: kinetics and compensation. J. Chem. Phys. 93, 1418–1429 (1990).
Chorkendorff, I. & Niemansverdriet, J. W. in Concepts of Modern Catalysis and Kinetics Ch. 2 (Wiley-VCH, 2003).
Temel, B., Meskine, H., Reuter, K., Scheffler, M. & Metiu, H. Does phenomenological kinetics provide an adequate description of heterogeneous catalytic reactions. J. Chem. Phys. 126, 204711 (2007).
Atkins, P. & de Paula, J. Atkins’ Physical Chemistry 8th edn (Oxford Univ. Press, New York, 2006).
Laidler, K. J. Chemical Kinetics 3rd edn (Prentice Hall, 1987).
Bond, G. C. Rosa, F. C. & Short, E. L. Kinetics of hydrolysis of carbon tetrachloride by acidic solids. Appl. Catal. A. Gen. 329, 46–57 (2007).
Temkin, M. Relation between the apparent and the true activation energy of heterogeneous reactions. Acta Physicochim. URSS 2, 313–316 (1935).
Yelon, A., Movaghar, B. & Crandall, R. S. Multi-excitation entropy: its role in thermodynamics and kinetics. Rep. Prog. Phys. 69, 1156–1194 (2006) and references therein.
Estrup, P. J., Greene, E. F., Cardillo, M. J. & Tully, J. C. Influence of surface phase transitions on desorption kinetics: the compensation effect. J. Phys. Chem. 90, 4099–4104 (1986).
Crihan, D. et al. Stable Deacon process for HCl oxidation over RuO2 . Angew. Chem. Int. Ed. 47, 2131–2134 (2008).
Zweidinger, S. et al. Reaction mechanism of the oxidation of HCl over RuO2(110). J. Phys Chem. C 112, 9966–9969 (2008).
López, N., Gómez-Segura, J., Marín, R. P. & Pérez-Ramírez, J. Mechanism of HCl oxidation (Deacon process) over RuO2 . J. Catal. 255, 29–39 (2008).
Studt, F. et al. Volcano relation for the Deacon process over transition-metal oxides. ChemCatChem 2, 98–102 (2010).
Hevia, M. A. G., Amrute, A. P., Schmidt, T. & Pérez-Ramírez, J. Transient mechanistic study of the gas-phase HCl oxidation to Cl2 on bulk and supported RuO2 catalysts. J. Catal. 276, 141–151 (2010).
Teschner, D. et al. An integrated approach to Deacon chemistry on RuO2-based catalysts. J. Catal. 285, 273–284 (2012).
Pérez-Ramírez, J. & Kondratenko, E. V. Evolution, achievements, and perspectives of the TAP technique. Catal. Today 121, 160–169 (2007).
Gleaves, J. T., Yablonsky, G., Zheng, X. L., Fushimi, R. & Mills, P. L. Temporal analysis of products (TAP)—recent advances in technology for kinetic analysis of multi-component catalysts. J. Mol. Catal. A 315, 108–134 (2010).
Revay, Z. et al. In situ determination of hydrogen inside a catalytic reactor using prompt γ activation analysis. Anal. Chem. 80, 6066–6071 (2008).
Teschner, D. et al. The roles of subsurface carbon and hydrogen in palladium-catalyzed alkyne hydrogenation. Science 320, 86–89 (2008).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integration. Phys. Rev. B 13, 5188–5192 (1976).
Acknowledgements
The authors acknowledge support from ETH Zurich, BMBF Project 033R018A, Bayer MaterialScience, the ICIQ Foundation, MICINN (CTQ2009-07753/BQU), BSC-RES, the EU FP7 NMI3 Access Programme, a NAP VENEUS grant (OMFB-00184/2006) and the cooperation project between the Fritz-Haber Institute and the former Institute of Isotopes founded by the MPG. K. Honkala is thanked for valuable suggestions.
Author information
Authors and Affiliations
Contributions
H.S and R.S. carried out and evaluated catalytic experiments to derive the Constable–Cremer relation. M.G.H. and J.P.R. performed and evaluated the TAP measurements. G.N.L. and N.L. carried out DFT calculations, microkinetic simulations and model system analysis. D.T., R.F., A.K.G. and L.Sz. performed and evaluated PGAA measurements. R.S. provided valuable suggestions for interpreting the results. D.T., J.P.R. and N.L. contributed to writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 813 kb)
Supplementary information
Supplementary movie 1 (MP4 28981 kb)
Supplementary information
Supplementary movie 2 (MP4 28672 kb)
Rights and permissions
About this article
Cite this article
Teschner, D., Novell-Leruth, G., Farra, R. et al. In situ surface coverage analysis of RuO2-catalysed HCl oxidation reveals the entropic origin of compensation in heterogeneous catalysis. Nature Chem 4, 739–745 (2012). https://doi.org/10.1038/nchem.1411
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1411
This article is cited by
-
Ion solvation kinetics in bipolar membranes and at electrolyte–metal interfaces
Nature Energy (2024)
-
Molecular-level insights into the electronic effects in platinum-catalyzed carbon monoxide oxidation
Nature Communications (2021)
-
Determination of the acidic properties of carboxylated carbocatalysts in an acid-catalyzed ring-opening reaction using kinetic profiling
Nano Research (2017)
-
Fifteen years of success: user access programs at the Budapest prompt-gamma activation analysis laboratory
Journal of Radioanalytical and Nuclear Chemistry (2016)
-
Expanding and Reducing Complexity in Materials Science Models with Relevance in Catalysis and Energy
Topics in Catalysis (2014)