Abstract
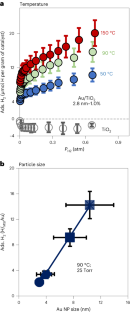
Hydrogen spillover involves the migration of H atom equivalents from metal nanoparticles to a support. While well documented, H spillover is poorly understood and largely unquantified. Here we measure weak, reversible H2 adsorption on Au/TiO2 catalysts, and extract the surface concentration of spilled-over hydrogen. The spillover species (H*) is best described as a loosely coupled proton/electron pair distributed across the titania surface hydroxyls. In stark contrast to traditional gas adsorption systems, H* adsorption increases with temperature. This unexpected adsorption behaviour has two origins. First, entropically favourable adsorption results from high proton mobility and configurational surface entropy. Second, the number of spillover sites increases with temperature, due to increasing hydroxyl acid–base equilibrium constants. Increased H* adsorption correlates with the associated changes in titania surface zwitterion concentration. This study provides a quantitative assessment of how hydroxyl surface chemistry impacts spillover thermodynamics, and contributes to the general understanding of spillover phenomena.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Raw data are available through ScholarSphere, Penn State’s open access repository at https://scholarsphere.psu.edu/ or available from the authors upon reasonable request. The atomic coordinates of the models in DFT calculations of charge density difference and spin density are provided in Supplementary Data 1.
References
Karim, W. et al. Catalyst support effects on hydrogen spillover. Nature 541, 68–71 (2017).
Hülsey, M. J., Fung, V., Hou, X., Wu, J. & Yan, N. Hydrogen spillover and its relation to hydrogenation: observations on structurally defined single-atom sites. Angew. Chem. Int. Ed. 61, e202208237 (2022).
Prins, R. Hydrogen spillover. Facts and fiction. Chem. Rev. 112, 2714–2738 (2012).
Joo, J. B. et al. Promotion of atomic hydrogen recombination as an alternative to electron trapping for the role of metals in the photocatalytic production of H2. Proc. Natl Acad. Sci. USA 111, 7942–7947 (2014).
Primo, A., Corma, A. & García, H. Titania supported gold nanoparticles as photocatalyst. Phys. Chem. Chem. Phys. 13, 886–910 (2011).
Panayotov, D. A. & Morris, J. R. Surface chemistry of Au/TiO2: thermally and photolytically activated reactions. Surf. Sci. Rep. 71, 77–271 (2016).
Chen, X., Liu, L., Yu, P. Y. & Mao, S. S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331, 746–750 (2011).
Lu, Y. et al. Self-hydrogenated shell promoting photocatalytic H2 evolution on anatase TiO2. Nat. Commun. 9, 2752 (2018).
Lucci, F. R. et al. Selective hydrogenation of 1,3-butadiene on platinum-copper alloys at the single-atom limit. Nat. Commun. https://doi.org/10.1038/ncomms9550 (2015).
Darby, M. T., Stamatakis, M., Michaelides, A. & Sykes, E. C. H. Lonely atoms with special gifts: breaking linear scaling relationships in heterogeneous catalysis with single-atom alloys. J. Phys. Chem. Lett. 9, 5636–5646 (2018).
O’Connor, C. R. et al. Facilitating hydrogen atom migration via a dense phase on palladium islands to a surrounding silver surface. Proc. Natl Acad. Sci. USA 117, 22657–22664 (2020).
Mori, K. et al. Hydrogen spillover-driven synthesis of high-entropy alloy nanoparticles as a robust catalyst for CO2 hydrogenation. Nat. Commun. 12, 3884 (2021).
Li, Y. & Yang, R. T. Significantly enhanced hydrogen storage in metal–organic frameworks via spillover. J. Am. Chem. Soc. 128, 726–727 (2006).
Esposito, D. V., Levin, I., Moffat, T. P. & Talin, A. A. H2 evolution at Si-based metal–insulator–semiconductor photoelectrodes enhanced by inversion channel charge collection and H spillover. Nat. Mater. 12, 562–568 (2013).
Kumaravel, V., Mathew, S., Bartlett, J. & Pillai, S. C. Photocatalytic hydrogen production using metal doped TiO2: a review of recent advances. Appl. Catal. B 244, 1021–1064 (2019).
Sampath, A. et al. Spectroscopic evidence for the involvement of interfacial sites in O–O bond activation over gold catalysts. ACS Catal. 12, 9549–9558 (2022).
Cargnello, M. et al. Control of metal nanocrystal size reveals metal–support interface role for ceria catalysts. Science 341, 771–773 (2013).
Frey, H., Beck, A., Huang, X., Bokhoven, J. A. V. & Willinger, M. G. Dynamic interplay between metal nanoparticles and oxide support under redox conditions. Science 376, 982–987 (2022).
Rolison, D. R. et al. Power of aerogel platforms to explore mesoscale transport in catalysis. ACS Appl. Mater. Interfaces 12, 41277–41287 (2020).
Sankar, M. et al. Role of the support in gold-containing nanoparticles as heterogeneous catalysts. Chem. Rev. 120, 3890–3938 (2020).
Saavedra, J., Doan, H. A., Pursell, C. J., Grabow, L. C. & Chandler, B. D. The critical role of water at the gold–titania interface in catalytic CO oxidation. Science 345, 1599–1602 (2014).
Yuan, W. et al. In situ manipulation of the active Au–TiO2 interface with atomic precision during CO oxidation. Science 371, 517–521 (2021).
Green, I. X., Tang, W., Neurock, M. & Yates, J. T. Jr. Spectroscopic observation of dual catalytic sites during oxidation of CO on a Au/TiO2 catalyst. Science 333, 736–739 (2011).
Corma, A. & Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 37, 2096–2126 (2008).
Hashmi, S. K. & Hutchings Graham, J. Gold catalysis. Angew. Chem. Int. Ed. Engl. 45, 7896–7936 (2006).
Zhang, Y., Cui, X., Shi, F. & Deng, Y. Nano-gold catalysis in fine chemical synthesis. Chem. Rev. 112, 2467–2505 (2012).
Ojeda, M. & Iglesia, E. Formic acid dehydrogenation on Au-based catalysts at near-ambient temperatures. Angew. Chem. Int. Ed. 48, 4800–4803 (2009).
Rodriguez, J. A. et al. Activity of CeOx and TiOx nanoparticles grown on Au(111) in the water–gas shift reaction. Science 318, 1757–1760 (2007).
Shekhar, M. et al. Size and support effects for the water–gas shift catalysis over gold nanoparticles supported on model Al2O3 and TiO2. J. Am. Chem. Soc. 134, 4700–4708 (2012).
Mitsudome, T. & Kaneda, K. Gold nanoparticle catalysts for selective hydrogenations. Green. Chem. 15, 2636–2654 (2013).
Sault, A. G., Madix, R. J. & Campbell, C. T. Adsorption of oxygen and hydrogen on gold(110)-(1 × 2). Surf. Sci. 169, 347 (1986).
Whittaker, T. et al. H2 oxidation over supported Au nanoparticle catalysts: evidence for heterolytic H2 activation at the metal–support interface. J. Am. Chem. Soc. 140, 16469–16487 (2018).
Sravan Kumar, K. B., Whittaker, T. N., Peterson, C., Grabow, L. C. & Chandler, B. D. Water poisons H2 activation at the Au–TiO2 interface by slowing proton and electron transfer between Au and titania. J. Am. Chem. Soc. 142, 5760–5772 (2020).
Mahdavi-Shakib, A., Rich, L. C., Whittaker, T. N. & Chandler, B. D. Hydrogen adsorption at the Au/TiO2 interface: quantitative determination and spectroscopic signature of the reactive interface hydroxyl groups at the active site. ACS Catal. 11, 15194–15202 (2021).
Mahdavi-Shakib, A. et al. Kinetics of H2 adsorption at the metal–support interface of Au/TiO2 catalysts probed by broad background IR absorbance. Angew. Chem. Int. Ed. 60, 7735–7743 (2021).
Honkala, K. et al. Ammonia synthesis from first-principles calculations. Science 307, 555–558 (2005).
Rekharsky, M., Inoue, Y., Tobey, S., Metzger, A. & Anslyn, E. Ion-pairing molecular recognition in water: aggregation at low concentrations that is entropy-driven. J. Am. Chem. Soc. 124, 14959–14967 (2002).
Hartshorn, H., Pursell, C. J. & Chandler, B. D. Adsorption of CO on supported gold nanoparticle catalysts: a comparative study. J. Phys. Chem. C 113, 10718–10725 (2009).
Campbell, C. T., Sprowl, L. H. & Árnadóttir, L. Equilibrium constants and rate constants for adsorbates: two-dimensional (2D) ideal gas, 2D ideal lattice gas, and ideal hindered translator models. J. Phys. Chem. C 120, 10283–10297 (2016).
Campbell, C. T. & Sellers, J. R. V. The entropies of adsorbed molecules. J. Am. Chem. Soc. 134, 18109–18115 (2012).
Campbell, C. T. & Sellers, J. R. V. Enthalpies and entropies of adsorption on well-defined oxide surfaces: experimental measurements. Chem. Rev. 113, 4106–4135 (2013).
Savara, A., Schmidt, C. M., Geiger, F. M. & Weitz, E. Adsorption entropies and enthalpies and their implications for adsorbate dynamics. J. Phys. Chem. C 113, 2806–2815 (2009).
Collinge, G. et al. Effect of collective dynamics and anharmonicity on entropy in heterogeneous catalysis: building the case for advanced molecular simulations. ACS Catal. 10, 9236–9260 (2020).
Vannice, M. A., Hyun, S. H., Kalpakci, B. & Liauh, W. C. Entropies of adsorption in heterogeneous catalytic reactions. J. Catal. 56, 358–362 (1979).
Spreafico, C., Karim, W., Ekinci, Y., van Bokhoven, J. A. & VandeVondele, J. Hydrogen adsorption on nanosized platinum and dynamics of spillover onto alumina and titania. J. Phys. Chem. C 121, 17862–17872 (2017).
Yun, T. Y. & Chandler, B. D. Surface hydroxyl chemistry of titania- and alumina-based supports: quantitative titration and temperature dependence of surface Brønsted acid–base parameters. ACS Appl. Mater. Interfaces 15, 6868–6876 (2023).
Luetzenkirchen, J. & Finck, N. Treatment of temperature dependence of interfacial speciation by speciation codes and temperature congruence of oxide surface charge. Appl. Geochem. 102, 26–33 (2019).
Beaumont, S. K., Alayoglu, S., Specht, C., Kruse, N. & Somorjai, G. A. A nanoscale demonstration of hydrogen atom spillover and surface diffusion across silica using the kinetics of CO2 methanation catalyzed on spatially separate Pt and Co nanoparticles. Nano Lett. 14, 4792–4796 (2014).
Setvin, M. et al. Methanol on anatase TiO2 (101): mechanistic insights into photocatalysis. ACS Catal. 7, 7081–7091 (2017).
Acknowledgements
The authors gratefully acknowledge the Department of Energy Basic Energy Sciences Program (DE-SC0022053 and DE-SC0016192) for primary support of this work. Preliminary experiments were supported by the National Science Foundation (CBET-1803769, 1803808 and 2102430) and the Research Corporation for Science Advancement. The computational work was completed with resources provided by the Research Computing Data Core at the University of Houston. We thank M. Janik at Penn State for invaluable discussions and T. Xie for his assistance in collecting TEM data.
Author information
Authors and Affiliations
Contributions
Conceptualization: B.D.C., T.N.W. and A.M.-S. Formal analysis: A.M.-S., T.N.W., T.Y.Y., S.W., K.B.S.K. and L.C.R. Funding acquisition: B.D.C., L.C.G. and R.M.R. Investigation: A.M.-S., T.N.W., T.Y.Y., L.C.R., S.W. and K.B.S.K. Methodology: A.M.-S., T.N.W., T.Y.Y. and S.G. Project administration: B.D.C. Supervision: B.D.C., A.M.-S. and L.C.G. Visualization: A.M.-S., T.N.W., T.Y.Y. and S.W. Writing—original draft: B.D.C. and A.M.-S. Writing—review and editing: T.N.W., R.M.R., L.C.G., S.W. and K.B.S.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Charles Campbell, Shuai Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Discussion, Figs. 1–19, Tables 1–7 and References.
Supplementary Data 1
This document contains the atomic coordinates of the models in DFT calculations of charge density difference and spin density.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahdavi-Shakib, A., Whittaker, T.N., Yun, T.Y. et al. The role of surface hydroxyls in the entropy-driven adsorption and spillover of H2 on Au/TiO2 catalysts. Nat Catal 6, 710–719 (2023). https://doi.org/10.1038/s41929-023-00996-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-00996-3
This article is cited by
-
Constructing regulable supports via non-stoichiometric engineering to stabilize ruthenium nanoparticles for enhanced pH-universal water splitting
Nature Communications (2024)
-
Engineering the Interface Between Au Nanoparticles and CoO-Ov to Enhance the Catalytic Performance of 5-Hydroxymethylfurfural (HMF) to 2,5-Dimethylfuran (DMF)
Chemical Research in Chinese Universities (2024)